 |
 |
- Search
| J Neurosonol Neuroimag > Volume 12(1); 2020 > Article |
|
Abstract
Posterior reversible encephalopathy is an uncommon clinical and radiological syndrome that is attributed to disruption of the blood-brain barrier. Various conditions, such as an abrupt drop in intracranial pressure, are related to this syndrome. A 71-year-old woman showed altered mental status after lumbar interbody fusion with laminectomy. An imaging study of the brain revealed findings suggestive of posterior reversible encephalopathy and intracranial hypotension concomitantly. After the primary closure, her mental status and brain imaging findings showed improvement.
Posterior reversible encephalopathy syndrome (PRES) is a clinicoradiological syndrome with reversible neurologic deficits, such as visual field defect, seizure, headache, and decreased consciousness, and characteristic brain image of vasogenic edema in the subcortical white matter of bilateral posterior regions.1 High blood pressure is a well-known evocative condition of PRES, but PRES can develop even in patients with a normotensive status because of infection, autoimmune disease, cytotoxic agents, anti-angiogenic agents, or renal failure.2 Here, we report a case of PRES due to intracranial hypotension.
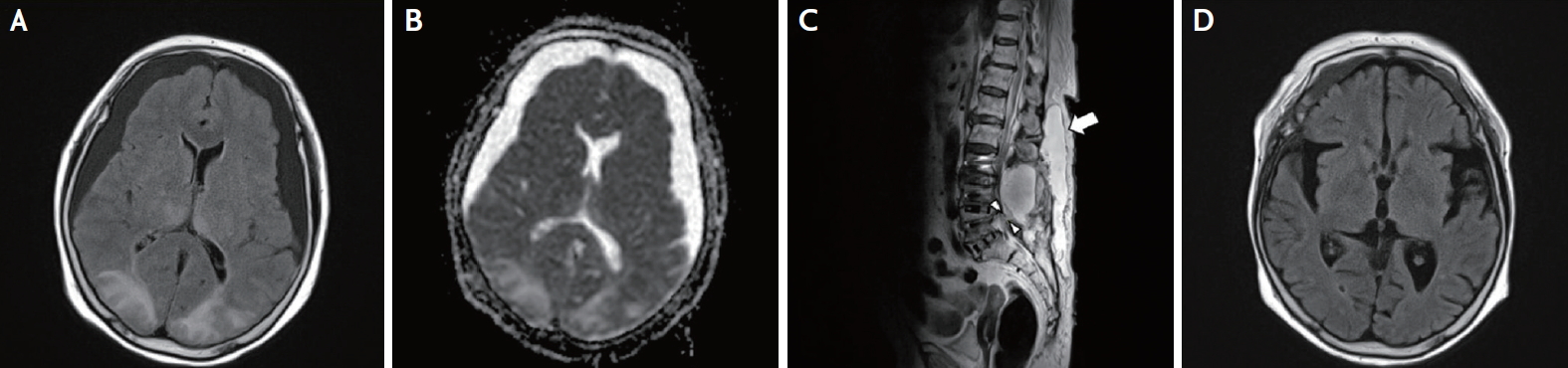
A 71-year-old woman with a history of hypertension was referred to the emergency room for dyspnea and altered mental status. She had undergone posterior lumbar interbody fusion with laminectomy in the region from the third lumbar to sacral vertebrae 9 days before. She complained of dyspnea 2 days before, during postoperative care, and showed a mildly drowsy mental status 1 day before. There was no medical record of high blood pressure or other neurologic deficits, such as headache or visual disturbance from previous hospital visits. Her blood pressure at the emergency room was 156/67 mmHg, and there was no definite evidence of infection. She was admitted for treatment of pulmonary embolism at the department of pulmonology. There was no significant change in her blood pressure during the course of acute management of pulmonary embolism. Her mental status changed to deeply drowsy with no other definite neurologic deficits after two days. She underwent brain magnetic resonance imaging (MRI), which showed symmetric high signal intensity in bilateral parietooccipital lobes, frontal convexity, thalamus, and cerebellum on fluid-attenuated inversion recovery and T2-weighted images, suggesting a high possibility of PRES. MRI also showed bilateral subdural effusion and mild sagging of the brain with compression of the diencephalon, which are compatible with intracranial hypotension. There were no features suggestive of venous thrombosis. Her blood pressure was within the normal range during hospitalization, and there were no causative factors, such as cytotoxic medication, renal failure, or sepsis. Lumbar spine MRI revealed cerebrospinal fluid (CSF) leakage with defects of the supraspinous ligament in the laminectomy bed and subcutaneous layers at the L1ŌĆōL3 level. Primary closure was performed, and her mental status improved. Follow-up MRI performed 1 month later showed complete recovery of PRES and absorption of bilateral subdural effusion with a normalized brain structure (Fig. 1).
Two mainstays of explanation for PRES are failure of the cerebral arterial autoregulatory system and endothelial dysfunction.3 An abrupt surge in blood pressure provokes not only an increase in the cerebral perfusion pressure but also forced vasodilation of the intracranial vessels, which can cause vasogenic edema with an increment in intravascular hydrostatic pressure. Along with high blood pressure, endothelial dysfunction due to the use of cytotoxic agents or systemic disease, which disrupt the blood-brain barrier, induces secretion of inflammatory cytokines. Excretion of vasoactive substances enhances the permeability of the endothelium, which aggravates vasogenic edema.2
In addition, PRES due to intracranial hypotension is attributed to abnormal flow of the venous system.4,5 According to the Monro-Kellie doctrine, the intracranial volume consists of three components: brain, blood, and CSF. The brain parenchyma is less flexible, so changes in blood volume are inevitable in alteration of the CSF volume to maintain the normal intracranial pressure.6 It is reflected in MRI findings with abnormal dural enhancement and signs of venous distension.7
Although rare, there are several case reports of PRES resulting from intracranial hypotension, including spontaneous intracranial hypotension, as an etiology (Table 1). Most patients completely recover with the treatment of CSF leakage or conservative care for PRES. Our patient was the first described case of PRES secondary to intracranial hypotension in Korea. Particularly, our case is not associated with other causative factors, such as pre-eclampsia, eclampsia, or cerebrovascular events. Our case demonstrated neurologic improvement and radiological complete remission after primary closure of the depletive site. Hence, clinicians should consider the probability of PRES in newly appeared neurologic deterioration of patients with intracranial hypotension.
Fig.┬Ā1.
Brain and lumbar spine magnetic resonance imaging suggesting posterior reversible encephalopathy syndrome due to intracranial hypotension. (A) Subdural effusion in the bilateral frontal areas and high signal intensities in the bilateral thalamus and occipitoparietal areas on fluid-attenuated inversion recovery. (B) Hyperintense lesions on apparent diffusion coefficient suggesting vasogenic edema. (C) Fluid collection in the subcutaneous layer of the 1st to 3rd lumbar regions (white arrow), and dural and supraspinous ligament defects in the 4th to 5th lumbar areas with a 10-cm diameter of fluid collection (arrow heads). No contrast enhancement in the surrounding connective tissues and a clear wall margin suggesting less possibility of infection. (D) Complete resolution of subdural effusion and hyperintense lesions after primary closure of the depletive site.
Table┬Ā1.
Cases of PRES secondary to intracranial hypotension with no other causative factors*
| Case | Age/sex | Clinical manifestation |
Image suggesting |
Etiology | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|
| Intracranial hypotensionŌĆĀ | PRESŌĆĪ | ||||||
| Pugliese et al. | 41/F | Headache and tonic-clonic seizure | Yes | Yes | Epidural tap for C-sec | Blood patch | No neurologic deficit |
| Mild left motor syndrome, mild right anisocoria and rapid deterioration of the mental status. | |||||||
| Doherty et al. | 19/F | Headache, blurred vision, ’¼éashing lights and seizure | No | Yes | Epidural tap for C-sec | Anticonvulsant | No neurologic deficit |
| Rajan et al. | 38/F | Headache and generalized tonic-clonic seizure | No | Yes | Spinal anesthesia for C-sec | IV Magnesium sulfate | No neurologic deficit |
| Grelat et al. | 69/F | Right hemiplegia, confusion, right side eyeball deviation and head version and cortical blindness | No | Yes | Ventriculo-peritoneal shunt | Antiepileptics and calcium channel blockers | Difficulties with executive functions |
| Antiplatelet | |||||||
| Shunt control | |||||||
| Matsui et al. | 68/F | Comatose mental status with status epilepticus occurred after surgery | No | Yes | Cystoperitoneal shunt | Anticonvulsant | No neurologic deficit |
| Hammad et al. | 72/M | Headache, transient vision loss, deterioration of mental status and tonic-clonic seizure | Yes | Yes | CSF leakage after spinal fusion | Anticonvulsant | No neurologic deficit |
| Santillan et al. | 65/F | Headache and progressive altered mental status | Yes | Yes | None | Blood patch | No neurologic deficit |
PRES; posterior reversible encephalopathy syndrome, RCVS; reversible cerebral vasoconstriction syndrome, FLAIR; fluid-attenuated inversion recovery.
* Other causative factors indicate hypertension, RCVS, post-partum state, (pre-) eclampsia, intracranial hemorrhage.
REFERENCES
1. Ollivier M, Bertrand A, Claren├¦on F, Gerber S, Deltour S, Domont F, et al. Neuroimaging features in posterior reversible encephalopathy syndrome: a pictorial review. J Neurol Sci. 2017;373:188-200.


2. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914-925.


3. Euser AG, Cipolla MJ. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension. 2007;49:334-340.


4. Hammad T, DeDent A, Algahtani R, Alastal Y, Elmer L, Medhkour A, et al. Posterior reversible encephalopathy syndrome secondary to CSF leak and intracranial hypotension: a case report and literature review. Case Rep Neurol Med. 2015;2015:538523.




5. Santillan A, Aamodt W, Bhavaraju-Sanka R. Pearls & Oysters: spontaneous intracranial hypotension and posterior reversible encephalopathy syndrome. Neurology. 2016;86:e55-e57.









