 |
 |
- Search
| J Neurosonol Neuroimag > Volume 14(1); 2022 > Article |
|
Abstract
Background
Atherogenic index of plasma (AIP) is an index representing the lipid profile of atherogenic dyslipidemia and has been shown to be associated with various atherosclerosis, metabolic diseases, and cardiovascular diseases. However, studies in patients with cerebrovascular disease are still insufficient. In this study, we evaluated the association between AIP and early recurrent ischemic lesions (ERILs) in patients with acute ischemic stroke (AIS).
Methods
We included consecutive patients with AIS between 2010 and 2016. ERILs were defined as new diffusion-weighted imaging lesions outside the initial symptomatic lesion area. AIP was calculated using the following formula: AIP=log (triglyceride [mg/dL]/high-density lipoprotein cholesterol [mg/dL]). For subgroup analysis according to stroke mechanism, participants were classif ied into the following four groups: intracranial-large artery atherosclerosis (LAA), extracranial-LAA, cardioembolism, and cryptogenic stroke.
Results
A total of 182 patients with AIS were evaluated (median age: 72 years, median initial NIHSS score: 4 [2-13]). In the multivariable analysis, AIP (adjusted odds ratio [aOR], 3.73; 95% confidence interval [CI], 1.01-13.82) was closely associated with ERIL after adjusting confounders. Age and initial NIHSS score were also related to ERIL. Among the four stroke mechanisms, only extracranial-LAA showed significant differences in AIP values between patients with and without ERIL (p=0.028).
Stroke recurrence in patients with ischemic stroke is clinically important because it increases the risk of subsequent disability and mortality.1 However, it is difficult to differentiate between initial neurological deficit and clinical worsening in patients with acute ischemic stroke (AIS), even if a well-trained neurologist conducts a daily neurological examination.1-3 Therefore, it is reported that the clinical recurrence rate after AIS is only less than 5%.2
However, since the development of diffusion-weighted imaging (DWI) technique sensitive to ischemic lesion, it has been reported that early radiological recurrence occurs quite frequently.1,4 These occurrences of new lesions called early recurrent ischemic lesions (ERILs) are so frequent that they occur in up to 40% of patients with AIS during the first week.2,4,5 Although most of ERILs are asymptomatic, they are considered to have similar pathologies as an index stroke or clinical recurrence.5,6 Therefore, several studies have been conducted so far to prevent the clinical recurrence of ischemic stroke by using ERIL, which is easy to analyze due to its high frequency, as a surrogate marker.1,4,7
Atherogenic dyslipidemia is a lipid profile represented by high triglyceride, low high-density lipoprotein (HDL) cholesterol, and small/dense low-density lipoprotein (LDL) cholesterol.8-10 These lipoproteins are known to cause various vascular complications including atherosclerosis.8,9 Therefore, as well as simply lowering LDL cholesterol, improving this lipid profile will also important in preventing the occurrence and worsening of cerebrovascular diseases.11 Atherogenic index of plasma (AIP) is an index that reflects such atherogenic dyslipidemia status as a single number.12 AIP has demonstrated a close association with atherosclerosis, metabolic diseases, and cardiovascular diseases in previous studies.13-15 However, there are still not sufficient studies on ischemic stroke patients.16
In this study, we evaluated the association between AIP and ERIL occurrence in patients with AIS. In addition, by performing subgroup analysis according to the mechanism of AIS, we explored which stroke mechanism AIP affects to cause ERIL.
From a consecutive registry at Seoul Metropolitan Government-Seoul National University Boramae Medical Center, we included patients with AIS who underwent follow-up magnetic resonance imaging (MRI) within 7 days of admission between January 2010 and December 2016 (n=279). Then, we additionally excluded participants with the following conditions: 1) time delay of >7 days from symptom onset to admission, 2) conducted diagnostic or therapeutic neuro-intervention procedures (n=11), or 3) previous use of lipid-lowering agents before AIP measurements (n=61).7 Also, as in previous studies dealing with ERIL, we excluded patients with the following stroke mechanisms in consideration of the characteristics of ERIL: 1) small vessel occlusion (n=14), 2) other determined (n=7), and 3) more than two mechanisms (n=4).7 Finally, a total of 182 AIS patients were included in the final analyses.
All experiments were performed in accordance with the Declaration of Helsinki and relevant guidelines and regulations. All data related to this study are included in the main text and supplemental materials.
We conducted broad evaluations including age, sex, hypertension, diabetes, atrial fibrillation, current smoking, stroke mechanisms, initial stroke severity, and systolic and diastolic blood pressures.7 The initial stroke severity was rated daily using the National Institutes of Health Stroke Scale (NIHSS) score by well-trained neurologists not involved in this study.7 The stroke mechanism was determined according to the Trial of ORG 10172 in Acute Stroke Treatment Classification.17 The study population was classified into four groups as follows: intracranial-large artery atherosclerosis (IC-LAA), extracranial-large artery atherosclerosis (EC-LAA), cardioembolism (CE), and cryptogenic stroke.7 IC-LAA was defined when there was symptomatic intracranial atherosclerosis without evidence of EC-LAA or CE.7 On the other hands, EC-LAA was defined as having symptomatic extracranial atherosclerosis without IC-LAA or CE.7
Laboratory examinations were performed after at least 12 hours of overnight fasting, including glucose profiles, lipid profiles, white blood cell (WBC) counts, and high-sensitivity C-reactive protein (hs-CRP).7 AIP was calculated by taking the log transformation of the ratio of triglyceride and HDL cholesterol as follows: AIP=log (triglyceride [mg/dL]/HDL cholesterol [mg/dL]).12
All participants in this study performed brain MRI and magnetic resonance angiography (MRA) within 24 hours of admission using a 3.0-T MR scanner (Achieva 3.0T; Philips, Eindhoven, the Netherlands). The details of MRI scan acquisition were as follows: The thickness of basic slice=5.0 mm; DWI (repetition time [TR]/echo time [TE]=3,000/44 ms); T1-weighted images (TR/TE=500/11 ms); T2-weighted images (TR/TE=3,000/100 ms); T2 fluid-attenuated inversion recovery images (TR/TE=11,000/120 ms); and three-dimensional time of flight MRA (TR/TE=24/3.5 ms, slice thickness=1.2 mm).
ERIL was defined as a new DWI lesion developing outside the initial symptomatic lesion area and not visible in the initial MRI (Fig. 1).7 The enlargement of the initial lesion was not considered as ERIL, in the line with previous studies.7 ERIL was based on radiological findings without consideration of the presence of clinical symptoms.7 The radiological assessments were performed by two trained neurologists (K.W.N. and H.M.K.), and disagreements were resolved through discussions with a third rater (Y.S.L.).
Continuous variables with normal distributions were showed as the mean±standard deviation, while the others were presented as the median+interquartile range. Continuous variables with skewed data were transformed into a log scale. To evaluate the possible predictors of ERIL, univariate analyses were performed using the Student’s t-test or Mann-Whitney U test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables. The variables with p<0.10 were introduced into the multivariable logistic regression analysis. Considering the formula of AIP, total- or HDL cholesterol and triglyceride were not introduced to multivariable analysis together with AIP due to overlapping and interaction problems.
AIP is an index that is not yet familiar to neurologists. Therefore, we tried to show the characteristics of AIP by showing the association between AIP and various variables. A simple linear regression analysis was used for this analysis. In addition, subgroup analysis was performed according to the four types of stroke mechanisms. By statistically analyzing the difference in AIP values between patients with and without ERIL, we attempted to examine the meaningful effects of AIP on the stroke mechanisms. All statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA). In this study, all variables with p<0.05 were consider statistically significant.
A total of 182 patients with AIS were assessed. The median age of the study population was 72 (64-82) years, and the proportion of males was 58.2%. The median value of the initial NIHSS score was 4 (2-13). The median visit time for patients after symptom onset was 0 (0-1) days, and the time from initial MRI to follow-up MRI was 3 (1-5) days. ERIL occurred in 76 patients (41.8%), and the median AIP value was 0.32 (0.12-0.54). Details of other baseline characteristics are given in Table 1.
In our study population, AIP showed a positive correlation with male sex (p=0.046), LDL cholesterol (p=0.002), triglyceride (p<0.001), WBC counts (p=0.028), hs-CRP (p=0.001), and ERIL (p=0.035). On the other hand, it showed a negative correlation with AF (p=0.013) and HDL cholesterol (p<0.001). In terms of stroke mechanisms, AIP showed a positive correlation with IC- and EC-LAA (p<0.001 and p=0.014, respectively), and a negative correlation with CE (p=0.001) and cryptogenic stroke (p=0.006) (Table 2).
In univariate analysis, ERIL was associated with age, initial NIHSS score, fasting glucose, LDL cholesterol, and triglyceride. AIP remained at a level showing a statistically marginal trend with ERIL (p=0.055) (Table 3). In multivariable logistic regression analysis, AIP (aOR, 3.73; 95% CI, 1.01-13.82) was positively associated with ERIL after adjusting for confounders. Age (aOR, 1.03; 95% CI, 1.01-.06) and initial NIHSS score (aOR, 1.06; 95% CI, 1.01-1.11) were also correlated with ERIL, being independent from AIP (Table 4).
When comparing according to stroke mechanisms, statistically significant differences in AIP values were observed between patients with and without ERIL only in the EC-LAA group (p=0.028). Even in the IC-LAA group, patients with ERIL had higher AIP values than those without ERIL, but this was not statistically significant (Fig. 2).
We compared the prognosis during hospitalization between the patients with and without ERIL to confirm the clinical significance of ERIL. Patients with ERIL had more frequent END (34.2% vs. 18.9%, p=0.019) and higher discharge NIHSS scores (6 [2-16] vs. 2 [0-8], p<0.001) than those without ERIL. Patients with ERIL also had higher discharge mRS scores, but the difference was not statistically significant (Table 5).
In this study, we found that AIP was closely associated with ERIL in patients with AIS. This close association was particularly conspicuous in patients with AIS due to EC-LAA. AIP may be involved in the development of new stroke lesion by affecting atherosclerotic plaques.
The exact pathological mechanisms that explain the close association between AIP and ERIL are unclear. However, the authors can offer plausible hypotheses. In our data, AIP showed a positive correlation with IC- and EC-LAA stroke, whereas it showed a negative correlation with CE or cryptogenic stroke (Table 2). In addition, although not statistically significant, patients with ERIL mainly suffered stroke due to the LAA mech-anism (Table 3). In other words, it seems appropriate to think about the association of AIP with ERIL in relation to the mechanism of LAA stroke.
As mentioned earlier, AIP is a marker of atherogenic dyslipidemia.12 These triglyceride-rich lipoproteins and small/dense LDL cholesterol easily penetrate into the intima of the artery.18 The infiltrating lipoproteins are degraded by lipase to product free fatty acids and monoacylglycerol, which leads to low-grade inflammation.19,20 In addition, these types of lipoproteins are uptaken by macrophages to form foam cells, thereby increasing the instability of atherosclerotic plaques.21 Triglyceride-rich lipoprotein increases plasma viscosity, promotes factor VII clotting activity, and interferes with fibrinolysis, which can also cause thrombosis.18 Due to these mechanisms, artery-to-artery embolism or in situ thrombosis may occur from atherosclerotic plaques of the large artery, causing ERIL.
However, as shown in our Fig. 2, AIP showed a difference in association with ERIL in the IC-LAA group and the EC-LAA group. Although the IC-LAA group had a higher median AIP value than the EC-LAA group (0.49 [0.29-0.61] vs. 0.45 [0.20-0.64]), there was no significant difference with or without ERIL. According to previous studies, EC-LAA is mainly caused by the mechanism of artery-to-artery embolism, whereas IC-LAA is known to be caused by various different pathological mechanisms (e.g., in situ thrombosis, branch atheromatous disease, artery-to-artery embolism).22 Some of the various mechanisms of IC-LAA may not be affected by AIP values, thereby weakening their statistical significance. On the other hand, it can be interpreted that artery-to-artery embolism causing EC-LAA may relatively closely be related to AIP. Of course, our results may also be the result from the statistical influence caused by the small sample size.
ERIL means the development of a novel lesion, regardless of whether symptoms recur or worsen. Therefore, some of ERILs may have occurred naturally as a result of decomposition of a thrombus blocking blood vessels during the formation of the initial lesions, regardless of the actual recurrence.7 However, as shown in Table 5, patients who underwent ERIL had a markedly worse prognosis during hospitalization and at discharge. Therefore, it would be of clinical significance to classify high-risk groups using ERIL and provide intensive treatment for them.
Although we presented novel findings, several limitations should be considered in interpreting the results of this study. First, since this study is a retrospective cross-sectional study, we can only show association, but it does not imply causality. Second, because of the nature of the outcome variable in this study, we included only patients who underwent follow-up MRI in the analysis. Follow-up MRI is not routinely performed in all AIS patients, and is mainly performed when clinical worsening occurs. Therefore, selection bias is bound to occur to some extent, and our high ERIL frequency should be interpreted in consideration of this point. Third, we also excluded patients who were taking lipid-lowering agents from the analysis. Although this also can cause selection bias, the authors thought that it was right to exclude these patients from the analysis, because various effects could directly affect to AIP value according to the type, dose, and timing of the drugs. Fourth, the sample size for analysis was not large due to the application of relatively strict exclusion criteria. Our findings, which were relatively clear even with a small number of samples, should be confirmed in subsequent studies involving a larger number of patients. Lastly, among the components of constituting the AIP formula, HDL cholesterol did not have statistical significance in the univariate analysis process. Therefore, our results may also be interpreted that the effect of hypertriglyceridemia is greater than that of other lipoproteins constituting atherogenic dyslipidemia.
In conclusion, we found that AIP was closely related to ERIL in patients with AIS, especially EC-LAA stroke. Currently, in most stroke patients caused by LAA are taking statin from the beginning of hospitalization. However, despite taking statin, ERIL occurred in many patients, and AIP was more closely related to ERIL than LDL cholesterol, a well-known risk factor. In other words, it may be necessary to develop a new therapeutic target or therapeutic agent for atherogenic dyslipidemia. Of course, these results and interpretations of our study should be verified by further studies.
NOTES
Ethics statement
This study was approved by the Institutional Review Board (IRB) of the Seoul Metropolitan Government-Seoul National University Boramae Medical Center (IRB No. 06-2020-0043). The requirement to obtain informed consent from the participants was waived by the IRB since this study was designed as a retrospective study using anonymous and de-identified information.
Fig. 1.
Representative cases of early recurrent ischemic lesion. The figures are representative cases of the occurrence of ERIL (red arrow) caused by (A) intracranial and (B) extracranial large artery atherosclerosis. ERIL refers to a new DWI lesion that is not visible in the initial magnetic resonance imaging, and the enlargement of the initial DWI lesion is not included. ERIL; early recurrent ischemic lesion, DWI; diffusion-weighted imaging.

Fig. 2.
Comparisons of the atherogenic index of plasma according to the presence of ERIL by stroke mechanisms. AIP showed a significant difference according to the presence or absence of ERIL only in the EC-LAA patients (p=0.028). Even in the IC-LAA group, patients with ERIL had higher AIP than those without ERIL, but this was not statistically significant (p=0.272). AIP; atherogenic index of plasma, ERIL; early recurrent ischemic lesion, IC-LAA; intracranial-large artery atherosclerosis, EC-LAA; extracranial-large artery atherosclerosis, CE; cardioembolic.
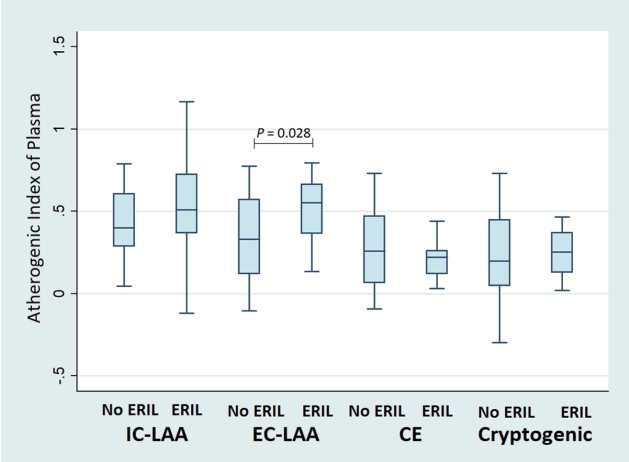
Table 1.
Baseline characteristics of the study population
Values are presented as number (%), median (interquartile range), or mean±standard deviation.
MRI; magnetic resonance imaging, LAA; large artery atherosclerosis, NIHSS; National Institutes of Health Stroke Scale, LDL; low-density lipoprotein, HDL; high-density lipoprotein, CRP; C-reactive protein, ERIL; early recurrent ischemic lesion.
Table 2.
Univariate linear regression analysis between the atherogenic index of plasma and clinical, laboratory, and radiological parameters
| β (95% CI) | p-value | |
|---|---|---|
| Age | -0.002 (-0.005 to 0.001) | 0.147 |
| Sex | 0.076 (0.001 to 0.152) | 0.046 |
| Hypertension | 0.024 (-0.058 to 0.105) | 0.569 |
| Diabetes | 0.037 (-0.047 to 0.121) | 0.385 |
| Atrial fibrillation | -0.103 (-0.184 to -0.022) | 0.013 |
| Smoking | 0.065 (-0.017 to 0.146) | 0.119 |
| Stroke mechanisms | ||
| Intracranial-LAA | 0.171 (0.085 to 0.257) | <0.001 |
| Extracranial-LAA | 0.108 (0.022 to 0.194) | 0.014 |
| Cardioembolic | -0.131 (-0.210 to -0.052) | 0.001 |
| Cryptogenic | -0.125 (-0.212 to -0.037) | 0.006 |
| Initial NIHSS score | -0.002 (-0.008 to 0.003) | 0.386 |
| Systolic blood pressure | 0.000 (-0.001 to 0.002) | 0.575 |
| Diastolic blood pressure | 0.001 (-0.001 to 0.003) | 0.493 |
| Hemoglobin A1c* | 0.281 (-0.259 to 0.821) | 0.305 |
| Fasting glucose* | 0.057 (-0.237 to 0.351) | 0.703 |
| Total cholesterol | 0.001 (0.000 to 0.002) | 0.066 |
| LDL cholesterol | 0.002 (0.001 to 0.003) | 0.002 |
| HDL cholesterol | -0.014 (-0.016 to -0.011) | <0.001 |
| Triglyceride* | 1.106 (1.003 to 1.209) | <0.001 |
| White blood cell | 0.014 (0.002 to 0.026) | 0.028 |
| High-sensitivity CRP* | 0.088 (0.038 to 0.138) | 0.001 |
| ERIL | 0.081 (0.006 to 0.156) | 0.035 |
Table 3.
Comparisons of baseline characteristics of patients with and without early recurrent ischemic lesion
Values are presented as number (%) or median (interquartile range).
ERIL; early recurrent ischemic lesion, MRI; magnetic resonance imaging, LAA; large artery atherosclerosis, NIHSS; National Institutes of Health Stroke Scale, LDL; low-density lipoprotein, HDL; high-density lipoprotein, CRP; C-reactive protein.
Table 4.
Multivariable logistic regression analysis of possible predictors for early recurrent ischemic lesion
| Crude OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
|---|---|---|---|---|
| Age | 1.03 (1.00-1.05) | 0.020 | 1.03 (1.00-1.06) | 0.036 |
| Initial NIHSS score | 1.06 (1.02-1.11) | 0.007 | 1.06 (1.01-1.11) | 0.022 |
| Diastolic BP | 1.01 (0.99-1.03) | 0.365 | 1.01 (0.99-1.03) | 0.361 |
| Fasting glucose* | 5.46 (0.53-55.92) | 0.153 | 3.54 (0.29-42.94) | 0.321 |
| LDL cholesterol | 1.01 (1.00-1.02) | 0.032 | 1.01 (1.00-1.02) | 0.075 |
| AIP | 3.56 (1.09-11.68) | 0.036 | 3.73 (1.01-13.82) | 0.049 |
Table 5.
Differences in subsequent prognosis between patients with and without early recurrent ischemic lesions
| No ERIL (n=106) | ERIL (n=76) | p-value | |
|---|---|---|---|
| END | 20 (18.9) | 26 (34.2) | 0.019 |
| Discharge NIHSS score | 2 (0-8) | 6 (2-16) | <0.001 |
| Discharge mRS | 0.068 | ||
| Good (0-2) | 70 (66.0) | 40 (52.6) | |
| Poor (3-6) | 36 (34.0) | 36 (47.4) |
REFERENCES
1. Kang DW, Han MK, Kim HJ, Sohn H, Kim BJ, Kwon SU, et al. Silent new ischemic lesions after index stroke and the risk of future clinical recurrent stroke. Neurology. 2016;86:277-285.



2. Kang DW, Latour LL, Chalela JA, Dambrosia J, Warach S. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol. 2003;54:66-74.


3. Nolte CH, Albach FN, Heuschmann PU, Brunecker P, Villringer K, Endres M, et al. Silent new DWI lesions within the first week after stroke. Cerebrovasc Dis. 2012;33:248-254.



4. Braemswig TB, Usnich T, Albach FN, Brunecker P, Grittner U, Scheitz JF, et al. Early new diffusion-weighted imaging lesions appear more often in stroke patients with a multiple territory lesion pattern. Stroke. 2013;44:2200-2204.


5. Kim WJ, Kim JH, Ko Y, Park JH, Yang MH, Jang MS, et al. Can early ischemic lesion recurrence on diffusion-weighted MRI affect functional outcome after acute ischemic stroke? J Clin Neurol. 2010;6:19-26.



6. Kang DW, Lattimore SU, Latour LL, Warach S. Silent ischemic lesion recurrence on magnetic resonance imaging predicts subsequent clinical vascular events. Arch Neurol. 2006;63:1730-1733.


7. Nam KW, Kwon HM, Lee YS. High triglyceride-glucose index is associated with early recurrent ischemic lesion in acute ischemic stroke. Sci Rep. 2021;11:15335.




8. Grundy SM. Small LDL, atherogenic dyslipidemia, and the metabolic syndrome. Circulation. 1997;95:1-4.


9. Musunuru K. Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Lipids. 2010;45:907-914.



10. Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol. 2020;75:2122-2135.



11. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350.

12. Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34:583-588.


13. Won KB, Heo R, Park HB, Lee BK, Lin FY, Hadamitzky M, et al. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis. 2021;324:46-51.

14. Ni W, Zhou Z, Liu T, Wang H, Deng J, Liu X, et al. Gender- and lesion number-dependent difference in “atherogenic index of plasma” in Chinese people with coronary heart disease. Sci Rep. 2017;7:13207.




15. Zheng Y, Li C, Yang J, Seery S, Qi Y, Wang W, et al. Atherogenic index of plasma for non-diabetic, coronary artery disease patients after percutaneous coronary intervention: a prospective study of the long-term outcomes in China. Cardiovasc Diabetol. 2022;21:29.




16. Liu H, Liu K, Pei L, Li S, Zhao J, Zhang K, et al. Atherogenic index of plasma predicts outcomes in acute ischemic stroke. Front Neurol. 2021;12:741754.



17. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35-41.


18. Hoshino T, Ishizuka K, Toi S, Mizuno T, Nishimura A, Wako S, et al. Prognostic role of hypertriglyceridemia in patients with stroke of atherothrombotic origin. Neurology. 2022;98:e1660-e1669.


19. Frohlich J, Dobiásová M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003;49:1873-1880.










