Recurrent Neuropsychiatric Systemic Lupus Erythematosus Presenting with Primary Central Nervous System Lymphoma
Article information
Abstract
We report the case of a systemic lupus erythematosus (SLE) patient who was diagnosed with lupus cerebritis, vasculitis, Epstein-Barr virus infection, and consequently, lymphoma. The patient presented to the emergency department with dysarthria, dysphagia, left-sided hemiparesis, and hypesthesia. Initial brain magnetic resonance imaging (MRI) revealed multiple high signal intensities in the cortex and subcortex. Intravenous methylprednisolone and immunoglobulin administration did not improve her symptoms. However, intravenous rituximab improved the parenchymal and angiographic abnormalities. One month later, she developed another acute left-sided hypesthesia. Brain MRI revealed multiple rim-enhancing lesions with perilesional edema, different from the previous lesion. Intravenous methylprednisolone and cyclophosphamide did not improve her symptoms, and she was finally diagnosed with primary central nervous system lymphoma after brain biopsy. Neurological symptoms improved after chemotherapy. Since the incidence of primary central nervous lymphoma is relatively high in SLE patients, careful diagnosis and accurate treatment are needed.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by chronic inflammation of various organs of the body. When SLE attacks the central nervous system (CNS), it is classified as neuropsychiatric SLE (NPSLE). However, NPSLE is a nonspecific term encompassing conditions that range from common and nonspecific symptoms, such as headache, cognitive dysfunction, and mood disorders, to specific disorders, such as cerebrovascular disease, cerebral vasculitis, demyelinating disease, and meningoencephalitis.1 Therefore, its diagnosis and treatment remain challenging.
Improvement in the treatment strategy of SLE patients has increased the survival rate of SLE patients these days.2 However, this has also led to an increased incidence of long-term complications such as infections and cardiovascular diseases, which affects patient morbidity and mortality.3 Moreover, the increased risk of malignancy, including lymphoma, by immune system dysregulation and chronic inflammation has been reported.4-6 Herein, we report the case of an NPSLE patient who was initially diagnosed with lupus cerebritis and vasculitis with subsequent successful treatment. A recurrent event with intractable NPSLE after 1 month was finally diagnosed as a primary CNS lymphoma.
CASE
A 53-year-old woman who had been diagnosed with SLE 7 years prior and used mycophenolate mofetil (500 mg/day) for 3 years visited the emergency department with transient dysarthria, dysphagia, left hemiparesis, and hypesthesia. Initial brain fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) revealed multifocal high signal intensities in the bilateral cortex and subcortex without angiographic abnormalities (Fig. 1A). Cerebrospinal fluid (CSF) showed normal opening pressure (110 mmH2O), increased white blood cell count (23 cells/mm3), and an increased protein level (82 mg/dL). Epstein-Barr virus (EBV) PCR was positive (titer: 32,500 copies/mL) in the CSF. On suspicion of lupus cerebritis and vasculitis, intravenous methylprednisolone (750 mg/day for 5 days) was initiated. Since her neurologic symptoms and brain image did not improve after high-dose steroid therapy, the EBV PCR titer was increased to 50,100 copies/mL, immunoglobulin (0.4 mg/kg/day for 5 days) and acyclovir (500 mg/day for 14 days) were initiated. Although her neurological symptoms improved, only mild headache remained. Brain MRI 25 days after admission revealed no improvement in FLAIR images, and luminal irregularity of intracranial arteries was newly developed (Fig. 1B). Thus, repeated intravenous methylprednisolone (750 mg/day for 5 days) and rituximab (1,000 mg/day) were administered. One week after the first dose of rituximab, the brain FLAIR MRI and angiographic abnormalities improved (Fig. 1C). Additional rituximab was administered and oral prednisolone was maintained at 60 mg/day and tapered to 10 mg/day every week. The patient was discharged without neurological symptoms.
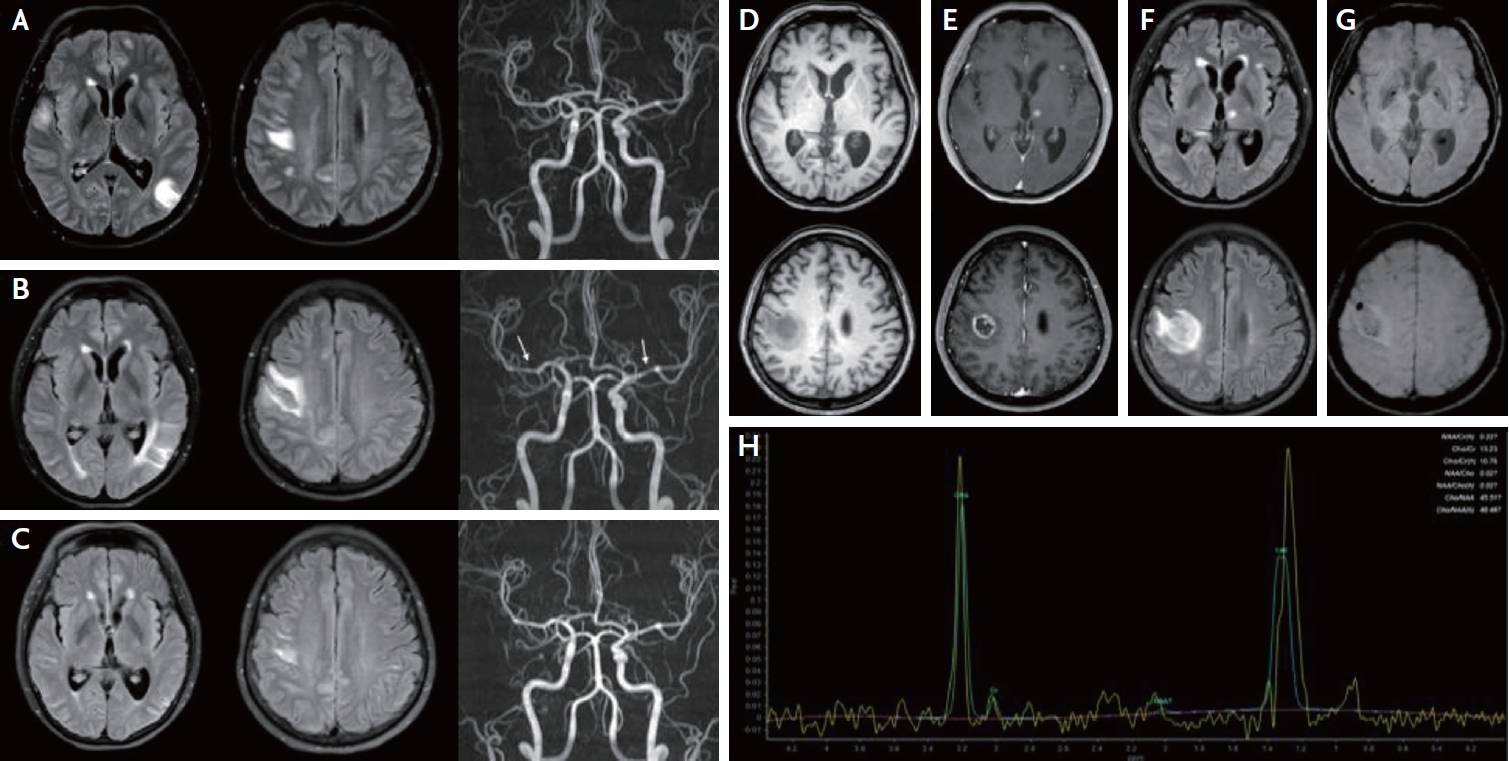
Brain magnetic resonance imaging, angiogram, and spectroscopy. Fluid attenuated inversion recovery (FLAIR) imaging at the first visit shows multifocal high signal intensities at both cerebral cortex and subcortex with normal angiography (A). After 1 month, high signal intensities on FLAIR are slightly increased with newly developed multifocal luminal irregularity (arrows) in the intracranial arteries (B). After rituximab treatment, parenchymal lesion and angiogram findings improved (C). On second admission, T1- (D) and T1-weighted enhance images (E) reveal multiple rim-enhancing lesions on the left thalamus, subcortex, and right subcortex. FLAIR image (F) shows perilesional edema and susceptibility weighted image (G) shows focal bleeding of the lesion. Magnetic resonance spectroscopy shows choline peak at right fronto-parietal enhancing lesion (H).
One month after discharge, she revisited the outpatient clinic of the neurology department with left facial hypesthesia, numbness of the left hand, and tremor. Brain MRI revealed multiple novel nodular enhancing lesions with perilesional edema and bleeding (Fig. 1D-G). CSF showed normal opening pressure (110 mmH2O), normal white blood cells (0 cells/mm3), mildly increased protein (48 mg/dL), and decreased EBV PCR titer (857 copies/mL). On suspicion of recurrent lupus cerebritis and vasculitis, intravenous methylprednisolone (750 mg/day for 5 days) and cyclophosphamide (500 mg/day) were administered. However, the patient showed neurological deterioration with left-sided motor weakness, memory impairment, confusion, and psychosis. Repeat MRI revealed worsening of the brain edema. Brain spectroscopy revealed a choline peak in the rim-enhancing lesion, suggesting a neoplastic lesion (Fig. 1H). Positron emission tomography revealed no primary cancer focus. Eventually, a brain biopsy was performed, and the patient was diagnosed with EBV-positive diffuse large B-cell lymphoma (Fig. 2). The patient’s neurological symptoms slightly improved after chemotherapy.
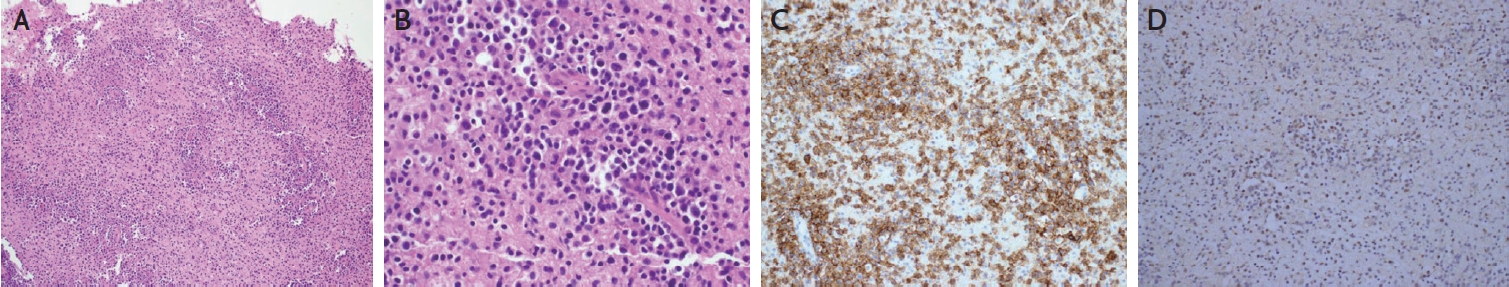
Representative photomicrograph of brain biopsy. At low magnification, large atypical lymphoid cell infiltrate brain parenchyma with an angiocentric pattern (A). At high magnification, the tumor cells comprise an immunoblastic cell population mixed with medium-to-large lymphoid cells (B). Tumor cells express the B-cell marker, CD20 in a diffuse pattern (C). In situ hybridization to Epstein- Barr virus (EBV)-encoded small RNA (EBER) indicates that tumor cells are positive for EBV (D). (A and B: H&E stain, ×100 and ×400, respectively; C: CD20 stain, ×200; D: EBER-In situ hybridization, ×200).
DISCUSSION
This case relates to the diagnosis of lupus-related cerebritis, vasculitis, EBV infection, and, ultimately, primary CNS lymphoma. The first brain image revealed lupus-related cerebritis and vasculitis; however, over time, the shape of the image changed to a tumorous appearance. Since, inflammatory SLE with vasculitis and cerebritis were initially suspected, high-dose corticosteroids, immunoglobulin, cyclophosphamide and rituximab were administered.1 Among the various treatments, intravenous methylprednisolone and rituximab were effective for the patient, while immunoglobulin and cyclophosphamide had insignificant treatment effects. This may account for the possible coexistence of vasculitis and lymphoma from the beginning. However, we believe that the patient’s first presentation was lupus vasculitis and cerebritis rather than primary CNS lymphoma because there were no enhancing lesions, and some edematous lesions completely disappeared and were initially improved by rituximab, which cannot cross the normal blood-brain barrier. The second attack was accompanied by numerous rim-enhancing lesions with perilesional edema, which was radiologically compatible with primary CNS lymphoma. She was finally diagnosed with EBV-positive diffuse large B-cell lymphoma through brain biopsy. The clinical and radiological findings improved with chemotherapy.
Lymphomas are a heterogeneous group of malignancies that arise from the clonal proliferation of B-cell, T-cell and natural killer cell subsets of lymphocytes at different stages of maturation.7 It has been reported that bidirectional relationship was present between SLE and non-Hodgkin lymphoma and incidence was high within the first year after the diagnosis of each disease.8 Although precise mechanisms were poorly understood, those two diseases shares genetics, chronic stimulation of immune system and disproportionate immune responses.9 Furthermore, treatment of SLE with immunosuppressive therapies such as mycophenolate mofetil, persistently activated immune system, chronic inflammation, viral infection including EBV and greater prevalence of traditional risk factors may explain high incidence of lymphoma in SLE patients.5,10 In our patient, primary diffuse large B-cell CNS lymphoma was occurred during 3 years of mycophenolate mofetil treatment and concomitant EBV infection which was similar with the characteristics of previous reports.10 Moreover, NPSLE was diagnosed before primary CNS lymphoma in some cases, as in our case.10 Since cancers such as lymphoma can develop in SLE patients undergoing mycophenolate mofetil treatment, prompt and accurate diagnosis with biopsy are necessary in such patients.
Notes
Ethics statement
Written informed consent was obtained from the patient.
Availability of Data and Material
None.
Sources of Funding
None.
Conflicts of Interest
No potential conflicts of interest relevant to this article was reported.
Acknowledgements
None.
