Cerebrovascular Function in Migraine Patients during, their Interictal Period, Compared to Normal Healthy Controls
Article information
Abstract
Background
Migraine is a debilitating neurovascular disorder which primarily impacts the working age population. The aim of the study was to determine if transient hemodynamic alterations during each migraine attack translates into cerebrovascular alterations, during migraineurs interictal period. A secondary hypothesis was to determine if there was any relationship between vascular hemodynamics and either the severity or frequency of migraine attacks.
Methods
We recruited 29 volunteers: 13 migraineurs (mean age 28±8.8 years; 12 female and 1 male) and 16 age-matched controls (mean age 26.6±6.9; 9 female and 7 male). Volunteers were classified as migraine sufferer or not (control group). All individuals underwent a detailed ophthalmic examination by a qualified optometrist and a Migraine Disability Assessment. Cerebral blood velocity measurements for the Middle Cerebral Artery (MCA) and Vertebral Artery (VA) were obtained using Colour Doppler Imaging using the Hitachi Aloka Noblus ultrasound system. The investigators were blind to the migraine diagnosis.
Results
During their interictal period migraineurs appear to have no significant difference in any hemodynamic parameter, for either their MCA and VA, when compared to normal healthy controls. Neither was a significant relationship between Migraine Disability Assessment Scores and any vascular parameter reported.
Conclusion
This study found nil cerebrovascular alterations which could be measured in migraineurs compared to normal healthy controls, during their interictal period.
INTRODUCTION
Migraine is a neurological disorder which is often associated with a range of debilitating symptoms. In the under fifties, it is the first cause of worldwide disability with an estimated prevalence of 14.7%.1 Attacks are most common in the working age population.2 The fiscal impact of migraine to the UK alone, is conservatively estimated at GBP 3.42 billion a year.3 Researchers have reported a positive correlation between the frequency of migraine and the levels of depression and anxiety.4 The prevalence of chronic migraine lies between 1.4 to 2.2%5 and is highest among middle-aged women.6 Sufferers are divided into migraine with or without aura. The lifetime prevalence of migraine without aura is double compared to those with aura.7 Vascular changes have an important role to play in migraine pathophysiology.8 Activation of the sensory neurons originating in the trigeminal ganglion, innervate blood vessels from both the meninges and the cerebral arteries via the release of vasoactive neuropeptides.9 This results in transient vasodilation, activating mechanical and chemical stimulation of the adjacent nociceptors, triggering migraine pain. Triptans are regularly used to treat migraine through vasoconstriction of meningeal vessels, decreased neurogenic inflammation, and reduced central nociception.10
Strong significant evidence shows that migraine attacks are accompanied by significant derangements in vascular function.11-14 The vascular theory states that during the aura phase of a migraine attack, there is an intracranial vasospasm resulting in a reduction in cerebral blood flow which continues to reduce whilst the headache develops.13 The early vasoconstrictive stage is followed by vasodilation of the meningeal blood vessels.13 which activates the trigeminal sensory nerves,15 causing pain. In more recent years there is evidence to suggest that the dilation of vessels, during a migraine attack, does not involve the intracranial vasculature or relate to the pain.11,12,14 These researchers believe that the vasodilation relates to, the extracranial terminal branches of the external carotid artery which instigates the pain.12,14 A theory which is further supported by the fact that migraine provoking agents have vascular altering properties and that the most successful medications (ergots and triptans) constrict abnormally dilated vessels.16
The prevalence of stroke and vascular disease is higher in patients diagnosed with migraine.17-19 Some researchers have reported that the risk of stroke is unexpectedly greater in younger female migraineurs.20,21 Normal tension glaucoma22 and migraine23 have a known female predominance. The reason behind this disparity is unknown however, some have postulated that the higher frequency of vasospasm24,25 and vascular disease19 found in females, also contributes towards the higher prevalence of normal tension glaucoma and migraine. Cerebral endothelial dysfunction has been documented in patients diagnosed with migraine.26,27 Vasodilation is endothelial dependent and can be measured via cerebrovascular reactivity. There is significant evidence to suggest that migraineurs have reduced cerebrovascular reactivity (poor auto-regulation), as the majority of studies recorded significantly lower breath holding index when their results were compared against normal healthy controls.28-32 Investigators have further postulated that reduced cerebrovascular reactivity, particularly in younger migraineurs, might explain in part the implausible relationship between stroke and younger migraineurs.31 Further clarification however is still desirable as one further study reported contradictory findings.33
The primary aim of the study was to determine if there are any vascular alterations in either the Middle Cerebral Artery (MCA) or the Vertebral Artery (VA), during the interictal period, which could be measured in migraineurs compared to normal healthy controls. A secondary hypothesis was to determine if there was any relationship between vascular hemodynamics, during the interictal period and either the severity or frequency of migraine attacks.
SUBJECTS AND METHODS
Participants aged between 18 and 45 years old, were recruited from December 2018 to May 2019. Otherwise healthy volunteers diagnosed with migraine and normal healthy controls were included in the study. Participants who were pregnant or presented with any ocular disease were excluded from the study. The study received ethical approval from the appropriate Institutional Review Board (City, University of London's ethics committee, Number Opt/PR/46). Prior to enrolment in the study written informed consent was obtained for all volunteers, following the tenets of the Declaration of Helsinki. An anonymous questionnaire (Supplementary Material) was completed by all volunteers. The questionnaire was developed according to the Headache International Society criteria34 and included a Migraine Disability Assessment Score (MIDAS). Volunteers were assigned to the control group if they had had less than 3 headaches in the past year and had not experienced a migraine. All volunteers were asked not to intake alcohol or caffeine and confirm that they had not suffered any migraine 24 hours before the test.
The following tests were carried out monocularly by all patients. Best-corrected visual acuity was recorded using a LogMAR chart (Thomson Software Solutions, Hatfield, UK). Ocular axial length was recorded using the Topcon Aladdin Optical Biometer and Corneal Topographer HW 3.0 (Tokyo, Japan), visual fields were examined using the Humphrey Field Analyzer (HFA-3) (Dublin, CA, USA). Objective refraction was carried out and intraocular pressure measurements were taken via the Topcon Auto Kerato-Refracto Tonometer (TRK-2P). Fundus photograph, peripapillary Retinal Nerve Fibre Layer thickness, Retinal Thickness, Ganglion Cell Complex, ranging from the inner-limiting membrane to the inner plexiform layer, and Choroidal Thickness were measured using the 3D OCT-1Maestro, Topcon, a Spectral Domain OCT (SD-OCT) device.
A different examiner who was an experienced sonographer (MC) recorded all the vascular measurements. The subject’s blood pressure height (cm) and weight (kg) were measured before taking the cerebral blood flow readings. Cerebral blood velocity measurements for the MCA and VA were obtained using Colour Doppler Imaging (CDI) using the Hitachi Aloka Noblus ultrasound system (Tokyo, Japan). Peak Systolic Velocity (PSV), End Diastolic Velocity (EDV), Mean Flow Velocity (MFV), Pulsatility Index (PI) and Resistivity Index (RI) were recorded bilaterally for each examined vessel. The standardised procedure of measuring cerebral blood flow via CDI was used. A Hitachi S211 5-1 MHz was gently applied to our participants trans-temporal and sub-occipital acoustic window while the patients were lying supine on their side and sitting upright on a chair, using a sterile ophthalmic coupling gel. This study has the advantage of ensuring that each investigator was blind to the diagnosis.
RESULTS
The population consisted of 13 migraine-suffering volunteers (mean age 28±8.8, 12 female and 1 male) and 16 normal healthy controls (mean age 26.6±6.9, 9 female and 7 male). Among the 13 participants suffering from migraine: 61.5% (8/13) had migraine with aura; 38.5% (5/13) had migraine with no aura. All data from both the migraine population and the normal healthy controls was initially tested via a Kolmogorov–Smirnoff test to determine if the data was normally distributed or not. Some were found to be normally distributed and the others were not. It was therefore necessary to use a combination of independent t-tests (parametric) and Mann–Whitney U tests (non-parametric) to investigate whether there was a significant difference between any blood flow parameters in the migraine population, during their interictal period compared to normal healthy controls.
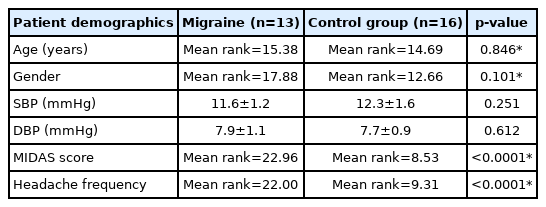
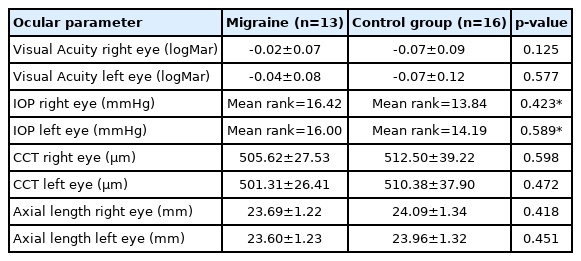
No patient had any ocular disease. Additionally, there was no significant difference between the migraine and control group in terms of either gender, distribution of age, systolic or diastolic blood pressure p>0.05 (Table 1), neither was there a significant difference between all ocular characteristics p>0.05 (Table 2). Unsurprisingly a significant difference was documented for MIDAS scores and headache frequency between the migraineurs and the normal healthy controls via Mann–Whitney U test p<0.0001.
No significant difference between EDV, PSV, MFV, RI & PI and either the VA or MCA between migraineurs and normal healthy controls via an independent t-test or the appropriate non-parametric equivalent was documented (Tables 3, 4). Finally, a Spearman’s Rank order correlation indicated no significant relationship between either MIDAS scores or the frequency of headache over a 3-month period for any of the vascular parameters recorded (p>0.05 for all).
DISCUSSION
Our study reports that migraineurs during their interictal period have no significant difference in any hemodynamic parameter, for either their MCA and VA, when compared to normal healthy controls. A recent meta-analysis also investigated these differences and reported higher PI in the posterior circulation of the migraine population, which was more evident when they included migraineurs diagnosed with aura.35 The authors documented that the mean resting blood flow velocity was higher in both the anterior and posterior circulation of migraine patients when it was compared against normal healthy controls. Closer inspection of their analysis reveals that although the study was extremely thorough in carrying out their systematic literature review. The researchers appear to have counted the data from several studies more than once and in some occasions up to four times. It is likely that this duplication of the data, skewed their results and artificially inflated precision, possibly leading to false conclusions.36 Their justification for dividing cerebrovascular functions into anterior and posterior circulations could also be made clearer. Closer evaluation of their forest plots suggests that the results from individual studies are quite variable, as opposing findings with wide confidence intervals are recorded. The literature review states that for the anterior and posterior circulation respectively, 86.7% (26/30) and 81.3% of studies (13/16) studies, found no significant difference between the mean blood velocity in migraineurs when results were compared to normal healthy controls. Conversely, outcomes from their meta-analysis suggests that resting blood velocity is significantly different in both circulations for the migraine population. These findings highlight the fact that double counting, may have led to conflicting views within the review and a false positive conclusion.
There was no relationship between either MIDAS scores or frequency of headache attack and any vascular constraint. Findings either suggest that there are no cerebral blood velocity alterations in migraine patients during an attack free period. An alternative explanation is that the present study did not employ sufficient numbers to detect these subtle differences. Recent evidence suggests that resting cerebral velocity alterations are at best contradictory, particularly when you have factored in studies which have carried out multiple statistical comparisons.28,29,32,33 Failing to correct for multiple comparisons significantly amplifies the probability of reporting a false positive finding. This suggests that this area of research would benefit from another meta-analysis which does not double count their data. The grouping of arteries should also be approached with caution.
In conclusion this study found nil cerebrovascular alterations which could be measured in migraineurs compared to normal healthy controls, during their interictal period. It must however be acknowledged that this study was limited by the small sample size included. A new systematic review and meta-analysis which includes resting and dynamic cerebral alterations in migraine patients compared to normal healthy controls is therefore needed.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.31728/jnn.2022.00122.
SUPPLEMENTARY MATERIAL
Participant Questionnaire
Notes
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of City, University of London. IRB No. Opt/PR/46. Written informed consent was obtained from each individual.
Availability of Data and Material
The data that support the findings of this study are available in the text.
Sources of Funding
We would like to thank City, University of London for their pump prime funds to purchase the transducer.
Conflicts of Interest
No potential conflicts of interest relevant to this article was reported.
Acknowledgements
The authors would like to thank Ms Teresa Paz Moraga for her help with the data collection. They would also like to thank Gill Harrison and Alison Harris for their technical support.