|
 |
- Search
| J Neurosonol Neuroimag > Volume 10(2); 2018 > Article |
|
Abstract
Background
Methods
Results
Conclusion
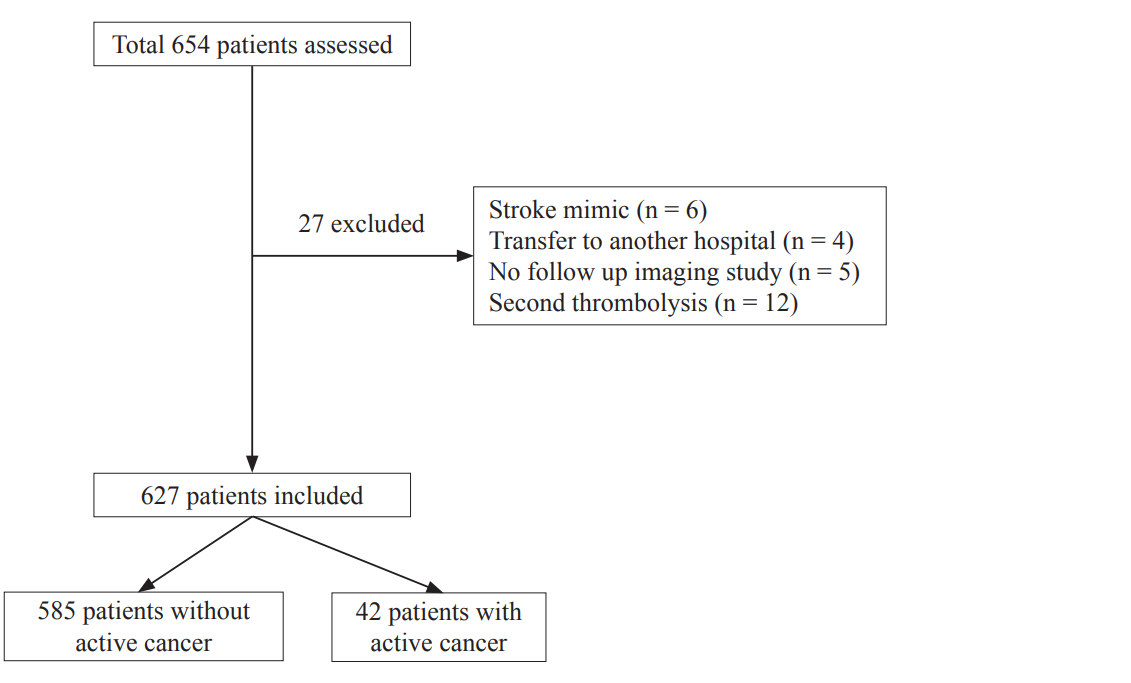
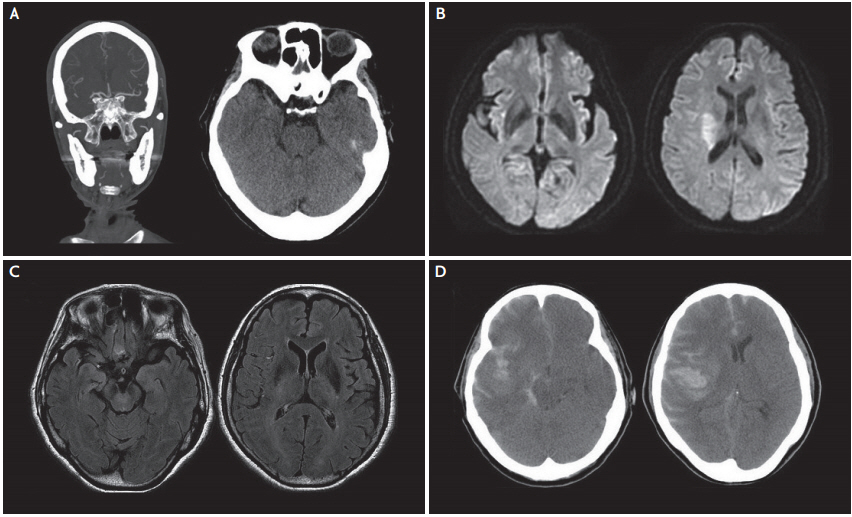
Fig.┬Ā2.
Table┬Ā1.
| Patients without cancer (n=585) | Patients with cancer (n=42) | p-value | |
|---|---|---|---|
| Male | 354 (60.5) | 28 (66.7) | 0.514 |
| Age | 67.33┬▒12.39 | 66.86┬▒8.65 | 0.808 |
| Smoking | 132 (22.6) | 7 (16.7) | 0.446 |
| Previous CAOD | 129 (22.1) | 9 (21.4) | 1 |
| Previous infarction | 87 (14.9) | 8 (19.0) | 0.502 |
| Previous hemorrhage | 22 (3.8) | 0 | 0.389 |
| Alcohol | 69 (11.8) | 4 (9.5) | 0.807 |
| Prior antiplatelet therapy | 203 (34.7) | 10 (23.8) | 0.178 |
| Prior anticoagulation | 65 (11.1) | 4 (9.5) | 0.810 |
| Hypertension | 403 (68.9) | 27 (64.3) | 0.606 |
| Diabetes mellitus | 159 (27.2) | 19 (45.2) | 0.014 |
| Hypercholesterolemia | 95 (16.2) | 9 (21.4) | 0.390 |
| Atrial fibrillation | 305 (52.1) | 14 (33.3) | 0.024 |
| High risk PCSE | 323 (55.2) | 15 (35.7) | 0.016 |
| CHF | 63 (10.8) | 6 (14.3) | 0.609 |
| Initial laboratory and clinical findings | |||
| ŌĆāGlucose, mmol/L | 7.783┬▒2.823 | 9.189┬▒3.229 | 0.002 |
| ŌĆāWBC, ╬╝L | 8,484.8┬▒2,745.2 | 9,177.1┬▒3,513.9 | 0.122 |
| ŌĆāHemoglobin, g/L | 137.2┬▒18.0 | 121.7┬▒19.5 | <0.001 |
| ŌĆāHematocrit | 0.403┬▒0.0051 | 0.362┬▒0.0059 | <0.001 |
| ŌĆāPlatelet, /╬╝L | 235,447.9┬▒67,314.0 | 263,547.6┬▒105,550.2 | 0.013 |
| ŌĆāPT, seconds | 11.90┬▒2.45 | 12.43┬▒2.72 | 0.183 |
| ŌĆāPT, INR | 1.02┬▒0.20 | 1.07┬▒0.23 | 0.173 |
| ŌĆāaPTT, seconds | 30.72┬▒9.34 | 28.94┬▒7.52 | 0.229 |
| ŌĆāD-dimer*, ng/mL | 1,948┬▒4,206 | 3,895┬▒6,476 | 0.143 |
| ŌĆāSBP, mmHg | 151.44┬▒29.72 | 137.05┬▒ 29.79 | 0.003 |
| ŌĆāDBP, mmHg | 84.83┬▒18.56 | 78.02┬▒13.54 | 0.020 |
| ŌĆāNIHSS | 14.71┬▒6.33 | 14.48┬▒6.77 | 0.816 |
| TOAST classificationŌĆĀ | 0.147 | ||
| ŌĆāCE | 307 (52.5) | 16 (38.1) | |
| ŌĆāLAA | 114 (19.5) | 13 (30.1) | |
| ŌĆāUE (2 or more) | 82 (14.0) | 3 (7.1) | |
| ŌĆāUE (negative) | 65 (11.1) | 9 (21.4) | |
| ŌĆāSVO | 3 (0.5) | 0 | |
| ŌĆāOD | 11 (1.9) | 1 (2.4) | |
| Outcomes | |||
| ŌĆāFavorable outcomeŌĆĪ | 292 (55.0) | 20 (50.0) | 0.622 |
| ŌĆā3 months mortalityŌĆĪ | 73 (13.7) | 11 (27.5) | 0.023 |
| ŌĆāAny HT | 231 (39.5) | 22 (52.4) | 0.106 |
| ŌĆāsICH | 88 (15.0) | 7 (16.7) | 0.823 |
Values are presented as mean┬▒standard deviation or number (%).
CAOD; coronary artery obstructive disease, PCSE; potential cardiac sources of embolism, CHF; congestive heart failure, WBC; white blood cell, PT; prothrombin time, INR; international normalized ratio, aPTT; activated partial thromboplastin time, SBP; systolic blood pressure, DBP; diastolic blood pressure, NIHSS; National Institutes of Health Stroke Scale, TOAST; trial of org 10172 in acute stroke treatment, CE; cardiogenic embolism, LAA; large artery atherosclerosis, UE; undetermined etiology, SVO; small vessel occlusion, OD; others-determined, HT; hemorrhagic transformation, sICH; symptomatic intracerebral hemorrhage.
Table┬Ā2.
Model 1; multiple regression analysis was performed with all variables. Model 2; multiple regression analysis was performed with selected variables whose p-value <0.2 from univariate analysis. Atrial fibrillation was excluded from the analysis because of the multicollinearity with PCSE.
ICH; intracerebral hemorrhage, OR; odds ratio, CI; confidence interval, CAOD; coronary artery obstructive disease, PCSE; potential cardiac sources of embolism, CHF; congestive heart failure, WBC; white blood cell, PT; prothrombin time, INR; international normalized ratio, aPTT; activated partial thromboplastin time, SBP; systolic blood pressure, DBP; diastolic blood pressure, NIHSS; National Institutes of Health Stroke Scale, TOAST; trial of org 10172 in acute stroke treatment, LAA; large artery atherosclerosis.
Table┬Ā3.
| Variable |
Model 1 |
Model 2 |
Model 3 |
|||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
| Sex (male reference) | 1.065 (0.530-2.140) | 0.859 | ||||
| Age | 1.035 (1.002-1.069) | 0.038 | 1.039 (0.998-1.082) | 0.063 | 1.021 (0.995-1.048) | 0.106 |
| Smoking | 0.261 (0.092-0.736) | 0.011 | 0.481 (0.210-1.101) | 0.083 | ||
| Previous CAOD | 1.163 (0.514-2.632) | 0.717 | ||||
| Previous infarction | 1.253 (0.550-2.854) | 0.591 | ||||
| Previous hemorrhage | 1.207 (0.293-4.972) | 0.794 | ||||
| Alcohol | 2.774 (0.986-7.809) | 0.053 | ||||
| Prior antiplatelet therapy | 0.818 (0.407-1.645) | 0.574 | ||||
| Prior anticoagulation | 0.223 (0.065-0.763) | 0.017 | ||||
| Cancer | 2.030 (0.691-5.968) | 0.198 | 3.668 (1.169-11.511) | 0.026 | 2.557 (1.076-6.073) | 0.033 |
| Hypertension | 0.655 (0.316-1.359) | 0.256 | ||||
| Diabetes mellitus | 0.975 (0.452-2.103) | 0.949 | ||||
| Hypercholesterolemia | 0.732 (0.279-1.922) | 0.526 | ||||
| High risk PCSE | 2.076 (0.745-5.782) | 0.162 | ||||
| CHF | 1.675 (0.702-3.994) | 0.245 | ||||
| Glucose | 0.912 (0.992-1.007) | 0.912 | ||||
| WBC | 1.000 (1.000-1.000) | 0.040 | ||||
| Hemoglobin | 0.937 (0.580-1.515) | 0.792 | 0.611 (0.487-0.767) | <0.001 | ||
| Hematocrit | 0.931 (0.787-1.101) | 0.403 | 0.912 (0.866-0.961) | 0.001 | ||
| Platelet | 1.000 (1.000-1.000) | 0.445 | ||||
| PT, INR | 6.293 (1.000-39.613) | 0.050 | ||||
| aPTT | 1.013 (0.969-1.059) | 0.572 | ||||
| SBP | 0.997 (0.984-1.011) | 0.675 | ||||
| DBP | 1.005 (0.985-1.025) | 0.637 | ||||
| NIHSS | 1.095 (1.037-1.157) | 0.001 | 1.078 (1.010-1.152) | 0.024 | 1.089 (1.037-1.142) | 0.001 |
| sICH | 11.631 (5.860-23.084) | <0.001 | 14.093 (6.197-32.046) | <0.001 | 11.447 (6.305-20.785) | <0.001 |
| TOAST (LAA reference) | 1.404 (0.491-4.015) | 0.526 | ||||
| D-dimer* | 1.000 (1.000-1.000) | 0.037 | ||||
Model 1; multiple regression analysis was performed with all variables. Model 2; multiple regression analysis was performed with selected variables whose p-value<0.2 from univariate analysis. Model 3; multiple regression analysis was performed with same variables as the model 2 except D-dimer.
OR; odds ratio, CI; confidence interval, CAOD; coronary artery obstructive disease, PCSE; potential cardiac sources of embolism, CHF; congestive heart failure, WBC; white blood cell, PT; prothrombin time, INR; international normalized ratio, aPTT; activated partial thromboplastin time, SBP; systolic blood pressure, DBP; diastolic blood pressure, NIHSS; National Institutes of Health Stroke Scale, sICH; symptomatic intracerebral hemorrhage, TOAST; trial of org 10172 in acute stroke treatment, LAA; large artery atherosclerosis.
Table┬Ā4.
| Patients without cancer (n=227) | Patients with cancer (n=13) | p-value | |
|---|---|---|---|
| Male | 148 (65.2) | 11 (84.6) | 0.150 |
| Age | 65.96┬▒13.03 | 68.77┬▒7.41 | 0.443 |
| Smoking | 54 (23.8) | 4 (30.8) | 0.739 |
| Previous CAOD | 55 (24.2) | 4 (30.8) | 0.740 |
| Previous infarction | 33 (14.5) | 3 (23.1) | 0.420 |
| Previous hemorrhage | 6 (2.6) | 0 | 1 |
| Alcohol | 32 (14.1) | 1 (7.7) | 0.701 |
| Prior antiplatelet therapy | 88 (38.8) | 5 (38.5) | 1 |
| Prior anticoagulation | 20 (8.8) | 0 | 0.395 |
| Hypertension | 155 (68.3) | 8 (61.5) | 0.761 |
| Diabetes mellitus | 67 (29.5) | 4 (30.8) | 1 |
| Hypercholesterolemia | 41 (18.1) | 3 (23.1) | 0.711 |
| Atrial fibrillation | 100 (44.1) | 2 (15.4) | 0.047 |
| High risk PCSE | 108 (47.6) | 3 (23.1) | 0.095 |
| CHF | 23 (10.1) | 1 (7.7) | 1 |
| Initial laboratory and clinical findings | |||
| ŌĆāGlucose, mmol/L | 7.689┬▒2.767 | 7.770┬▒2.295 | 0.921 |
| ŌĆāWBC, ╬╝L | 8,625.7┬▒2,738.1 | 9,646.2┬▒4,359.3 | 0.420 |
| ŌĆāHemoglobin, g/L | 139.1┬▒17.6 | 123.0┬▒17.4 | 0.001 |
| ŌĆāHematocrit | 0.408┬▒0.0050 | 0.363┬▒0.0052 | 0.002 |
| ŌĆāPlatelet, /╬╝L | 244,211.5┬▒72,960.0 | 232,384.6┬▒69,076.7 | 0.569 |
| ŌĆāPT, seconds | 11.74┬▒2.39 | 12.22┬▒2.39 | 0.451 |
| ŌĆāPT, INR | 1.00┬▒0.19 | 1.04┬▒0.18 | 0.326 |
| ŌĆāaPTT, seconds | 30.34┬▒4.51 | 30.86┬▒8.73 | 0.632 |
| ŌĆāD-dimer*, ng/mL | 2,535┬▒6,149 | 3,510┬▒4,485 | 0.283 |
| ŌĆāSBP, mmHg | 154.61┬▒30.08 | 140.62┬▒38.94 | 0.110 |
| ŌĆāDBP, mmHg | 87.31┬▒18.16 | 75.23┬▒10.41 | 0.001 |
| ŌĆāNIHSS | 11.94┬▒5.69 | 10.92┬▒4.70 | 0.529 |
| TOAST classification | 0.471 | ||
| ŌĆāCE | 109 (48.0) | 4 (30.8) | |
| ŌĆāLAA | 36 (15.9) | 2 (15.4) | |
| ŌĆāUE (2 or more) | 37 (16.3) | 2 (15.4) | |
| ŌĆāUE (negative) | 34 (15.0) | 5 (38.5) | |
| ŌĆāSVO | 3 (1.3) | 0 | |
| ŌĆāOD | 5 (2.2) | 0 | |
| Outcomes | |||
| ŌĆāFavorable outcomeŌĆĀ | 131 (66.2) | 6 (50.0) | 0.349 |
| ŌĆā3 months mortalityŌĆĀ | 16 (8.1) | 5 (41.7) | 0.003 |
| ŌĆāAny HT | 37 (16.3) | 7 (53.8) | 0.003 |
| ŌĆāsICH | 5 (2.2) | 4 (30.8) | 0.001 |
Values are presented as mean┬▒standard deviation or number (%).
IV; intravenous, rt-PA; recombinant tissue plasminogen activator, CAOD; coronary artery obstructive disease, PCSE; potential cardiac sources of embolism, CHF; congestive heart failure, WBC; white blood cell, PT; prothrombin time, INR; international normalized ratio, aPTT; activated partial thromboplastin time, SBP; systolic blood pressure, DBP; diastolic blood pressure, NIHSS; National Institutes of Health Stroke Scale, TOAST; trial of org 10172 in acute stroke treatment, CE; cardiogenic embolism, LAA; large artery atherosclerosis, UE; undetermined etiology, SVO; small vessel occlusion, OD; others-determined, HT; hemorrhagic transformation, sICH; symptomatic intracerebral hemorrhage.
REFERENCES
-
METRICS

-
- 5 Crossref
- Scopus
- 4,606 View
- 67 Download
- Related articles in AC