Transcranial Doppler Monitoring in Subarachnoid Hemorrhage
Article information
Abstract
Cerebral vasospasm and delayed cerebral ischemia are common complications associated with subarachnoid hemorrhage (SAH) outcomes. Various modalities can be used to evaluate and detect cerebral vasospasm after SAH. Transcranial Doppler (TCD) ultrasonography can be used as an appropriate bedside dynamic monitoring tool to screen for the presence and progression of cerebral vasospasm in patients with SAH. This article briefly reviewed the severity of SAH and pathophysiology of vasospasm. In addition, information regarding the use of TCD for monitoring cerebral vasospasm, several variables measured by TCD to monitor vasospasm detection, and differentiating vasospasm from physiological conditions in the clinical setting following SAH were presented in this article.
INTRODUCTION
Subarachnoid hemorrhage (SAH), especially aneurysmal SAH, is a devastating cerebrovascular disease with high morbidity and mortality and peaks between the ages of 50 and 60 years. The incidence of SAH is 9 to 23 persons per 100,000 population, with considerable variation in different regions worldwide.1-4 After initial treatments with surgical or endovascular methods, patients remain at risk of major complications, including symptomatic cerebral vasospasm and delayed cerebral ischemia (DCI), thus developing further morbidity and mortality associated with poor outcomes.3-9 Symptomatic vasospasm develops in 20% to 40% of patients with SAH, and 19% to 46% of these patients experience symptomatic DCI, with the incidence peaking in the second week after ictus.3-8,10,11 Therefore, cerebral vasospasm requires immediate detection and appropriate preventative or therapeutic interventions to prevent clinically significant DCI during the period of high risk of cerebral vasospasm using routine monitoring following SAH in the neurological intensive care unit (NICU).4,12,13 Transcranial Doppler (TCD) has been extensively used as an inexpensive and noninvasive tool that allows the sensitive evaluation for cerebral vasospasm after SAH.12,14-17 Moreover, the patient’s cerebral hemodynamics can be evaluated in real time, and the treatment effect on cerebral vasospasm can be confirmed through serial examinations using TCD monitoring at bedside in the NICU.
RADIOLOGICAL GRADING SCALES FOR SUBARACHNOID HEMORRHAGE
The Fisher scale, reported in 1980, is the initial and widely used scale for assessing the amount of SAH on the initial brain computed tomography (CT) within 5 days of SAH (Table 1, Fig. 1).18 The Fisher scale grades the amount of cisternal or sylvian blood without considering the concomitant intraventricular hemorrhage (IVH). In addition, Fisher’s four-grade scale scores indicate that higher scores, such as large amounts of bleeding, are associated with the risk of developing cerebral vasospasm. However, the Fisher scale was criticized because the grade 3 group showed the highest incidence of cerebral vasospasm compared to the other groups.18-20 In 2001, a modified Fisher scale (also known as the Claassen grading system) was proposed. The modified Fisher scale incorporates the presence or absence of IVH in the presence of blood in the subarachnoid space on the initial brain CT. The modified Fisher scale showed that the presence of IVH increased the risk of developing cerebral vasospasm, and this risk progressively increased with each grade of the modified Fisher scale (Table 1, Fig. 1).19,20 Therefore, the modified Fisher scale is a more reasonable radiological grading scale for predicting cerebral vasospasm and DCI after SAH.

Fisher scale and modified Fisher scale on brain CT in SAH. (A) Grade 1, no SAH; grade 2, diffuse or vertical thin SAH; grade 3, diffuse vertical thick SAH; and grade 4, diffuse or no SAH with IVH or intraparenchymal hemorrhage. (B) Grade 1, focal or diffuse thin SAH without IVH; grade 2, focal or diffuse thin SAH with IVH; grade 3, thick SAH without IVH; and grade 4, thick SAH with IVH. This figure was reproduced with permission from Frontera et al.49 CT; computed tomography, SAH; subarachnoid hemorrhage, IVH; intraventricular hemorrhage.
CEREBRAL VASOSPASM AFTER SUBARACHNOID HEMORRHAGE
Cerebral vasospasm is defined as narrowing of cerebral arteries after SAH, which often begins 2-4 days following SAH, and lasts for up to 21 days.6,7,13 Cerebral vasospasm can lead to decreased cerebral blood flow, resulting in DCI with neurological deficit related to prognosis in patients with SAH.6,10,13,21,22 Therefore, cerebral vasospasm must be treated as a clinical diagnosis along with radiological diagnosis, based on the neurological symptoms after SAH.6,14,21,22 Although the mechanisms for cerebral vasospasm are not completely clear, oxyhemoglobin, the breakdown products of blood cells, and other metabolites, including endothelin, can enter the vessel walls and result in biochemical reaction.6,10,22 This can lead to contraction of vascular smooth muscle cell, increase the contents of vasoconstriction, and impair the process of endothelin-dependent vasodilation and structural changes of vessels.6,10,22 These changes result in severe narrowing of the cerebral arteries and can increase vascular resistance with secondary decreases in cerebral blood flow.6,20 The most important risk factor of cerebral vasospasm is the volume of blood, as revealed on brain CT as a severe SAH. Moreover, poor neurological status on admission, age, smoking, hypertension, diabetes mellitus, and female sex associated with cerebral vasospasm in SAH.23,24 Angiographic vasospasm develops in approximately 70% of patients with SAH, which is detected within 48 h of SAH in each cerebral artery.3-8,6,15,21 The severity of vasospasm based on the angiographic findings of two-dimensional vessel diameters is categorized into three groups as follows: 1) mild, reduction <25% of vessel caliber; 2) moderate, reduction between 25% and 50%; and 3) severe, reduction >50%.6,15,21 Angiographic vasospasm is defined as moderate-to-severe arterial narrowing on digital subtraction angiography (DSA) without other causes. Radiological vasospasm often precedes clinical neurological deficits; thus, progressive vasospasm can be treated to prevent neuronal injury.21,25-27 Symptomatic and clinical vasospasms occur in 20-40% of patients, which are the development of new focal neurological signs or deterioration of neurological symptoms if the cause is cerebral ischemia attributable to cerebral vasospasm. Symptomatic vasospasm is associated with morbidity and mortality after SAH.13,21,26,27
CEREBRAL VASOSPASM MONITORING USING TCD IN SUBARACHNOID HEMORRHAGE
Although DSA is the gold standard for the diagnosis of cerebral vasospasm with higher sensitivity and specificity, DSA and CT angiography provide only snapshot information about cerebral vasospasm at the time of imaging.5,15,28 Moreover, they are invasive methods that require contrast administration, and risks are associated with the transportation of patients with SAH for performing these imaging examinations.15 However, TCD is a tool used extensively for the surveillance and monitoring of cerebral vasospasm in patients with SAH during the symptomatic period related to vasospasm and the pre-symptomatic period. The dynamic phenomenon of cerebral vasospasm can be monitored using TCD at the bedside in real time, and TCD is portable and a noninvasive method that is easy to repeat every day depending on the patients’ neurological symptoms.14,15 Therefore, TCD is recommended for monitoring the development and temporal course of cerebral vasospasm during its incidence periods.1,2,23
Furthermore, serial TCD monitoring during vasospasm therapy, including intra-arterial administration of nimodipine or oral nimodipine, can be helpful in evaluating the improvement of cerebral vasospasm based on the relative change of the velocities, as detected by TCD.29,30 Cerebral blood flow velocity increases and pressure decreases when blood flow moves from a larger diameter vessel to one with a smaller diameter with cerebral vasospasm, allowing the same volume of blood to pass through the vasospasm occurring in a narrow area, which can be described using the Bernoulli principle.14,26,31,32 The change in blood flow velocities measured by TCD directly reveals the hemodynamic phenomenon of vasospasm following SAH.14,26,31,32
When monitoring cerebral blood flow velocity, the peak systolic velocity (PSV) and end diastolic velocity (EDV) are measured from the waveform of the TCD. Cerebral vasospasm is usually evaluated as the mean flow velocity (MFV) of cerebral arteries, which is calculated using the formula MFV=(PSV+2×EDV)/3 (cm/s).14,26,31,32 The sensitivity or specificity of TCD in detecting cerebral vasospasm depends on cerebral arteries related to its collateral pattern.14,26,32,33 However, several patients’ physiological parameters, such as heart rate, blood pressure, hematocrit, and arterial carbon dioxide (CO2) tension, and physicians’ level of experience can affect TCD velocity measurements.14,26,32,33 Higher blood pressure and increased partial pressure of CO2 are reflected by increased MFV on TCD. Hematocrit level is inversely associated with MFV. If the hematocrit level decreases from 40% to 30%, the MFV increases by ~20%.34 In addition, there is a limitation because of the restriction of insonation secondary to inadequate acoustic windowing of the temporal bone in approximately 8-20% of patients. Therefore, these factors should be recorded using TCD monitoring to compare serial changes and provide context for interpretation.12,14
TCD CRITERIA FOR CEREBRAL VASOSPASM
1. Middle cerebral artery (MCA)
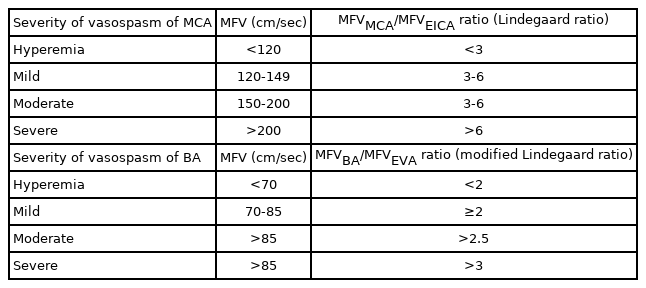
In general, the correlation between TCD findings and angiographic vasospasm is clinically reliable in the MCA with high sensitivity and specificity (sensitivity, 67-88%; specificity, 72-99%).35-37 In general, MFV <120 cm/s is a reliable predictor of the absence of cerebral vasospasm (94% negative predictive value), whereas an MFV of 120-200 cm/s indicates mild to moderate cerebral vasospasm, and an MFV >200 cm/s is graded as severe vasospasm (87% positive predictive value).12,14,15,21,27,32,35,38 Additionally, an MFV >50 cm/s in 24 hours may be a sign of the aggravation of cerebral vasospasm.39,40 However, it is important to differentiate between vasospasm and hyperemia related to physiological conditions that can influence blood flow velocity, such as autoregulation, hypertension, and hypervolemia. The Lindegaard ratio has been proposed to differentiate between cerebral vasospasm and hyperemic flow. The Lindegaard ratio is calculated using the MFV of the MCA and the ipsilateral extracranial internal carotid artery (EICA) as follows: Lindegaard ratio=MFVMCA /MFVEICA. A Lindegaard ratio of 3-6 is a sign of mild to moderate vasospasm, and a Lindegaard ratio >6.0 is an indication of severe vasospasm (Table 2, Figs 2, 3). A Lindegaard ratio <3.0 is suggestive of hyperemia or other physiological conditions, although blood flow velocity is increased (Fig. 2).12,14,15,17,25,41
Transcranial doppler discrimination of vasospasm and hyperemia. (A) TCD with increased systolic flow velocity, increased diastolic flow velocity, and loss of the dicrotic notch. Lindegaard ratio <3. These findings indicated hyperemia. (B) TCD with increased systolic flow velocity, increased diastolic flow velocity, and presence of a dicrotic notch. Lindegaard ratio >3. These findings indicated vasospasm. This figure was reproduced with permission from O’Brien et al.50 TCD; transcranial Doppler.

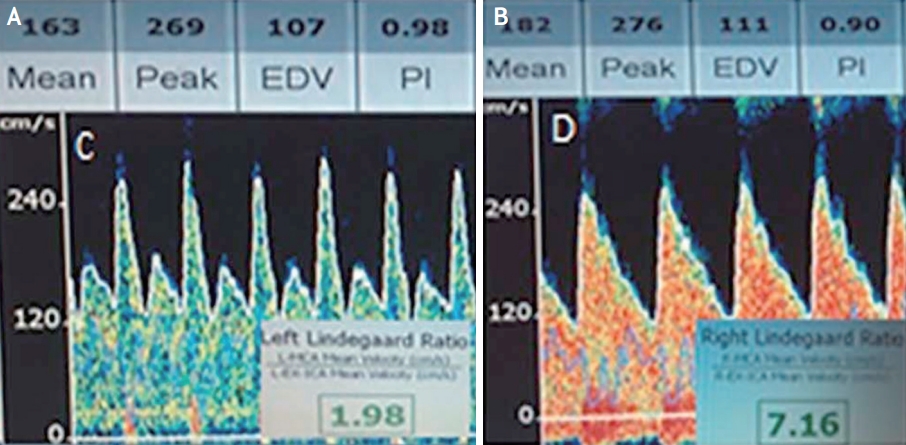
TCD monitoring of MCA vasospasm. (A) Subarachnoid hemorrhage on brain CT secondary to a ruptured aneurysm (arrow). Note the left frontal craniectomy (*). Transcranial doppler US images and spectral waveforms obtained in the left (B) MCA and ipsilateral (C) ICA show 150 cm/s MFV in the left (B) MCA and 13 cm/s MFV in the left ICA. Lindegaard ratio is 11.5, indicative of severe vasospasm. (D) Three-dimensional reconstruction from CT angiography depicts a severe stenosis of the left MCA (arrow). This figure was reproduced with permission from Kirsch et al.14 CT; computed tomography, US; ultrasonography, MCA; middle cerebral artery, ICA; internal carotid artery, MFV; mean flow velocity.
2. Anterior cerebral artery (ACA)
The usefulness of TCD as a screening tool for the diagnosis of ACA cerebral vasospasm is low because of its low sensitivity (18-50%) and specificity (~65%) due to the difficulty of insonation.14,15,38,42 To improve the accuracy of monitoring vasospasm in ACA using TCD, evaluating both ACAs on each side since the collateralization of the proximal ACAs by the prominent anterior communicating artery is recommended.43 Regarding the MFV value, an ACA MFV ≥120 cm/s has 18% sensitivity and 65% specificity to detect cerebral vasospasm in ACA.38,44 For ACA vasospasm, increasing MFV by 50% or 50 cm/s than baseline MFV within 24 hours is a more reliable predictive value (Fig. 4).5,28 Moreover, the loan hemispheric ratio (MFVACA/MFVEICA >4) can be used for the diagnosis of vasospasm in ACA (Table 3).26,42
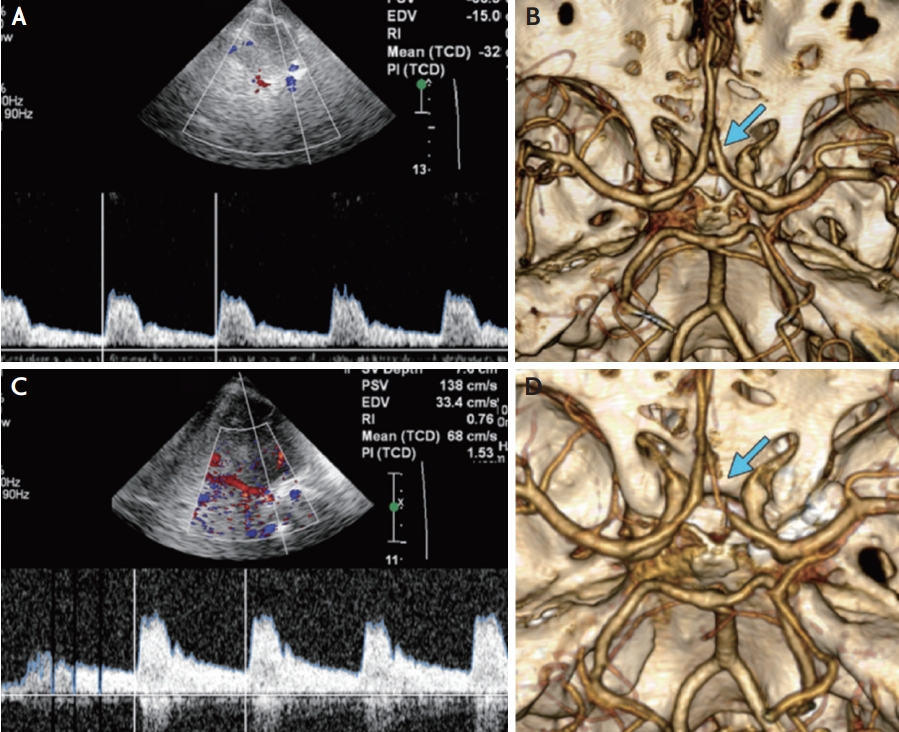
TCD monitoring of ACA vasospasm. (A) Transcranial Doppler US image shows normal MFV of 32 cm/s in the right ACA immediately after aneurysm rupture. (C) Follow-up transcranial duplex Doppler US image obtained after 10 days showed an increased MFV of 68 cm/s in the right ACA. The MFV is normal (68 cm/s) but has also increased since baseline (32 cm/s). Three-dimensional images from (B) baseline and (D) follow-up CT angiography demonstrating interval development of right ACA vasospasm (arrows). This figure was reproduced with permission from Kirsch et al.14 US; ultrasonography, ACA; anterior cerebral artery, MFV; mean flow velocity, CT; computed tomography.
3. Posterior cerebral artery (PCA)
The evaluation of cerebral vasospasm using TCD is limited by low sensitivity (42-48%) and specificity (69- 72%) in the PCA because of collateral flow and variation in the anatomical position of PCA.15,38,45 Previous studies reported that the MFV cutoff value for detecting cerebral vasospasm is >110 cm/s, with 55% positive predictive value and 78% negative predictive value in PCA (Table 3).12,14,15,38,45 However, serial TCD studies can be more important for the diagnosis of vasospasm in PCA after SAH.
4. Basilar artery (BA)
Although vasospasm in the posterior circulation has not been well studied, detection of BA vasospasm using TCD has good sensitivity (76.9%) and specificity (79.0%).12,15 In the BA, an MFV >85 cm/s is a sign of vasospasm. In addition, a BA MFV >115 cm/s is associated with the risk of DCI after SAH.12,15,46-48 For the diagnosis of vasospasm in BA, a modified Lindegaard ratio was proposed, which was analyzed using the MFV of the BA and MFV extracranial vertebral artery (EVA) as follows: modified Lindegaard ratio=MFVBA/MFVEVA. The MFVEVA is calculated using the averaged maximum MFVs from both VAs.46-48 A modified Lindegaard ratio >2 is a predictor of BA vasospasm, with 73% sensitivity and 80% specificity. Combining a modified Lindegaard ratio >3 with a BA MFV >85 cm/s is an indicator of BA narrowing >50% (sensitivity, 92%; specificity, 97%). The modified Lindegaard ratio can improve the accuracy of the diagnosis of BA vasospasm (Table 2).15,17,46-48
CONCLUSION
TCD is a reasonable and noninvasive tool used for bedside monitoring of cerebral hemodynamic changes in patients with SAH. Cerebral vasospasm is the main complication associated with DCI and the prognosis following SAH. Therefore, the routine daily measurement of TCD velocities in patients with SAH is recommended until the expected period of cerebral vasospasm for up to 21 days. TCD monitoring for detecting vasospasm in the MCA and BA is useful for detecting progressed vasospasm and deciding treatment plans earlier, although the evaluation of ACA and PCA vasospasm is limited. Therefore, TCD can become a primary screening and monitoring tool in the NICU for the evaluation and management of vasospasm secondary to SAH.
Notes
Ethics statement
The Institutional Review Board process and patient consents were not proceeded because of a review article.
Availability of Data and Material
The data supporting the findings of this study are available from the corresponding author (Pf. Tae Jung Kim) upon request.
Sources of Funding
This study was funded by the Seoul National University Hospital (No. 0320210350).
Conflicts of Interest
No potential conflicts of interest relevant to this article was reported.
Acknowledgements
None.