Use of a Balloon Catheter for Occlusion of Iatrogenic Direct Carotid-Cavernous Fistula Occurring during a Neurointerventional Procedure
Article information
Abstract
Neurointerventional procedures involving the anterior circulation, including aneurysm coiling, mechanical thrombectomy (MT), and intracranial angioplasty, can result in rare life-threatening complications such as iatrogenic carotid-cavernous fistulas (CCFs). Here, we describe the successful use of a balloon catheter for the occlusion of an iatrogenic CCF during neurointerventional procedures in a 78-year-old woman. The patient underwent successful MT for right middle cerebral artery occlusion; however, her symptoms worsened the day after the procedure; therefore, intracranial stenting was planned. An iatrogenic CCF that occurred during the neurointerventional procedure was successfully occluded using a balloon catheter. Neurointerventionalists should be aware of iatrogenic CCFs and be prepared to treat this rare complication. The double-lumen balloon catheter may be a useful tool for the transarterial treatment of iatrogenic CCFs.
Direct carotid-cavernous fistula (CCF) is a rare, life-threatening disorder in which a direct shunt arises between the intracavernous carotid artery and cavernous sinus. This condition usually occurs due to trauma or aneurysmal rupture and is rarely iatrogenic.1 Iatrogenic CCFs are described as a complication in approximately 0.8% of neurointerventional procedures that involve the anterior circulation, including mechanical thrombectomy (MT), intracranial angioplasty, and aneurysm coiling.2
Here, we report a case of iatrogenic CCF that occurred during a neurointerventional procedure as well as a treatment option for iatrogenic CCF.
CASE
A 78-year-old woman presented with left hemiplegia and recurrent transient dysarthria that had developed 2 hours and 2 weeks before presentation. She had no history of hypertension, diabetes, hyperlipidemia, or heart disease, except for a history of transarterial chemoembolization for hepatocellular carcinoma. Neurological examination revealed a left central-type facial palsy, left hemiplegia, asomatognosia, and visual extinction. The initial National Institutes of Health Stroke Scale (NIHSS) score was 13. Brain computed tomography angiography revealed an occlusion of the M1 segment of the right middle cerebral artery (MCA). As there were no contraindications for intravenous thrombolytic administration, intravenous alteplase was administered, and MT was planned for occlusion of the M1 segment of the right MCA. We performed Solumbra MT using a 9F Cello balloon guiding catheter (Medtronic, Dublin, Ireland), an ACE68 aspiration catheter (Penumbra, Alameda, CA, USA), and a Solitaire™ Platinum stent-retriever (Medtronic, Dublin, Ireland). Although stenosis remained in the M1 segment of the MCA, successful recanalization was achieved (modified thrombolysis in cerebral infarction 2b). After the procedure, visual extinction, hemiplegia, and dysarthria improved, and the patient’s NIHSS score decreased to 5. However, the hemiplegia and dysarthria worsened the day after the procedure. Conventional angiography revealed severe stenosis of the M1 segment of the right MCA; therefore, intracranial angioplasty and stenting were performed. We used an 8F shuttle guiding sheath (Cook Medical, Bloomington, IN, USA), 5F diagnostic catheter, and 0.035” wire. The guiding catheter can be difficult to deliver because of the severe tortuosity of the proximal internal carotid artery (ICA). The 0.035’’ wire was placed in the cavernous ICA, and while the guiding catheter was advancing, the tortuous ICA suddenly extended, and the wire penetrated the cavernous ICA. Rapid blood flow to both cavernous sinuses was observed in the arterial phase of the right internal carotid angiography performed after wire retrieval, which suggested a direct CCF (Fig. 1A, B). The shunted outflow drained into the internal jugular vein through the inferior petrosal sinuses on both sides. Angiography was performed 10 min later in anticipation of spontaneous occlusion of the iatrogenic CCF, but the shunted flow was sustained. To occlude the CCF, a balloon catheter (Scepter C, 4×10 mm, MicroVention Inc., Tustin, CA, USA) was placed in the cavernous ICA and inflated for 2 min at the site of the suspected penetration injury (Fig. 1C, D). The balloon was inflated twice by slightly moving the catheter back and forth. The shunt was no longer observed on the subsequent right internal carotid angiography (Fig. 2). Angioplasty and stenting were not performed for right MCA stenosis because of severe tortuosity of the proximal ICA. After three weeks, the patient was transferred to an outside hospital for rehabilitation.
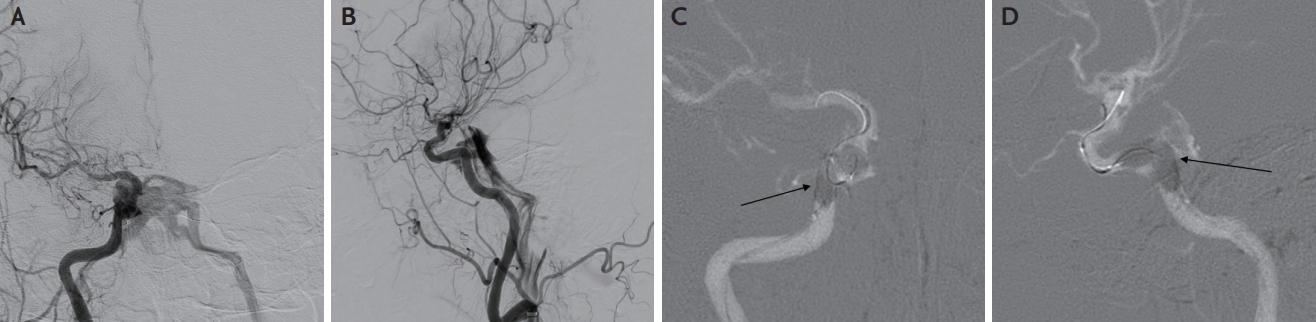
Digital subtraction angiography on hospital day 2. (A) Anteroposterior (AP) projection view and (B) lateral projection view of right internal carotid angiogram showing direct carotid-cavernous fistula. Shunted flow drained to both internal jugular veins via the inferior petrosal sinus. (C) AP projection view and (D) lateral projection view showing a balloon inflated in the right cavernous internal carotid artery. The black arrows of C, D indicate the inflated balloon.

Digital subtraction angiography after occlusion of iatrogenic carotid-cavernous fistula (CCF), and magnetic resonance angiography (MRA) followed up the day after the procedure. (A) Anteroposterior projection view and (B) lateral projection view of right internal carotid angiogram showing obliteration of the direct CCF. (C) MRA followed up the day after the procedure showed no recurrence of CCF.
DISCUSSION
The Barrow classification classifies CCFs into four types. Type A CCFs are direct connections between the ICA and cavernous sinuses, and types B, C, and D CCFs are all indirect dural shunts from the meningeal branches of the ICA, external carotid artery, or both.3 The etiology of direct Type A CCFs usually includes head trauma, Ehlers-Danlos syndrome type IV, ICA aneurysm rupture, or iatrogenic neurointerventional procedures.4 This case involved iatrogenic direct CCF caused by injury to the cavernous ICA via wire manipulation. Type A fistulas usually present with critical symptoms due to a high-flow shunt between the ICA and cavernous sinus. Patients may present with chemosis, diplopia, proptosis, ocular bruits, and increased intraocular pressure, which can lead to central retinal vein occlusion or optic neuropathy.4 However, in this case, the ocular symptoms were absent because there was no reflux into the superior ophthalmic vein and because the CCF was occluded within a short time using a balloon catheter.
In general, direct CCFs are unlikely to occlude spontaneously, and in approximately 20% of patients, emergency treatment may be required due to decreased visual acuity, increased intraocular pressure, and intracranial hemorrhage.5 However, the natural course of iatrogenic CCF during neurointerventional procedures is not well known. Ono et al.2 reported that iatrogenic CCF occurred in nine (0.8%) of 1,071 cases of transarterial endovascular treatment. Of these, only two cases of CCF spontaneously disappeared. The shunt flow was decreased by balloon inflation in one case, no additional treatment was required in one case, and five cases required transarterial coil embolization.
The treatment of CCF depends on the severity of clinical manifestations, angiographic architecture, and risk of intracranial hemorrhage. Almost all direct CCFs require treatment because they are unlikely to occlude spontaneously and have a high risk of intracranial hemorrhage or neurological deterioration. Various options are available for the treatment of CCFs, including surgery, radiosurgery, and endovascular treatment; however, endovascular treatment is preferred in most cases.5
Endovascular treatment options for direct CCFs are divided into transarterial and transvenous approaches. The transarterial approach includes transarterial stent-assisted coil embolization, transarterial stent deployment with a detachable balloon in the cavernous sinus, and transarterial coil embolization with liquid embolic materials such as n-butyl cyanoacrylate and ethylene-vinyl alcohol copolymer. This technique involves placing a microcatheter across the tear into the cavernous sinus and filling the sinus. During transarterial embolization, a temporary balloon may be placed in the cavernous segment of the ICA to protect the parent vessel and prevent migration of the embolic material into the distal intracranial circulation.5 Transvenous approach may be an option in treating iatrogenic CCFs that cannot be treated by a transarterial approach. Polytetrafluoroethylene-covered stents are therapeutic alternatives to treat iatrogenic CCF and cannot be successfully occluded with detachable balloons or coils.6 The transvenous route usually approaches the shunt involving the cavernous sinus through the inferior petrosal sinus (IPS) via the internal jugular vein. If the IPS is occluded or absent, access to the cavernous sinus can be obtained through the superior ophthalmic vein via the facial vein.5
Few reports are available on iatrogenic direct CCFs that occur during neurointerventional procedures. The natural course of CCFs is not well known, and further studies are needed. After occlusion of iatrogenic CCF, even in rare cases, CCF may recur, and follow-up imaging studies should be considered, as in our case.
In conclusion, with the increasing frequency of endovascular therapy for the treatment of acute ischemic stroke, neurointerventionalists should be aware of CCF and prepared to treat this rare potential complication. The double-lumen balloon catheter may be a useful tool for the transarterial treatment of iatrogenic CCFs.
Notes
Ethics statement
This case report was approved by the Research Ethics Committee of the Jeju National University Hospital (Approval No. JEJUNUH 2021-08-004). The IRB of Jeju National University Hospital waived the need for informed consent.
Availability of Data and Material
All data and materials related to this article are included in the main text.
Sources of Funding
None.
Conflicts of Interest
No potential conflicts of interest relevant to this article was reported.
Acknowledgements
None.
