Recurrent Cerebral Infarction due to Cerebral Vasculitis in a Patient with Ulcerative Colitis
Article information
Abstract
Vascular complications are extraintestinal manifestations of ulcerative colitis. Although there has been some controversy regarding the association between ulcerative colitis and stroke, hypercoagulability and systemic inflammation are thought to be possible factors contributing to stroke pathogenesis. Herein, we report a case of recurrent ischemic stroke with significant arterial stenosis. Cerebral vasculitis was suggested in high-resolution magnetic resonance vessel wall imaging (VWI) and marked improvement was confirmed by follow-up VWI after steroid treatment. This case suggests that VWI can be a useful tool to diagnose cerebral vasculitis and evaluate therapeutic effect.
Ulcerative colitis (UC), a chronic disorder of the colonic mucosa, is an inflammatory bowel disease (IBD).1 Extraintestinal manifestations could accompany UC, and more than half of the patients experience extraintestinal complications at least once in their lifetime. Various neurologic syndromes, including stroke, may occur in patients with UC, but the pathomechanism has not been identified. Thromboembolism and vasculitis are suggested as the causative factors of stroke in patients with UC.2 Confirming the pathophysiology of stroke is important for determining the treatment strategy. Recently, high-resolution magnetic resonance vessel wall imaging (VWI) has been helpful in improving our understanding of the intracranial arterial wall pathology, especially in cerebral vasculitis.3 Here, we report a case of recurrent ischemic stroke due to vasculitis confirmed by VWI in a patient with UC.
CASE
A 49-year-old man presented with transient left-sided weakness of sudden onset. His initial vital signs were as follows: blood pressure: 185/112 mmHg; heart rate: 80 beats/min; respiratory rate: 20/min; and body temperature: 36.5°C. Electrocardiography revealed normal sinus rhythm. The patient was diagnosed with polycystic kidney disease 13 years ago and underwent kidney transplantation (KTP) 3 years ago. A year after his KTP, he was diagnosed with post-transplant UC. Left side weakness improved within 5 minutes, but he suffered a transient left side weakness within 30 minutes on the same day. Diffusion-weighted imaging (DWI) showed diffusion restriction lesions in the right insular cortex and parietal subcortex (posterior portion of the right splenium) (Fig. 1A), and magnetic resonance angiography (MRA) showed stenosis in the A2 segment of the right anterior cerebral artery (ACA) and the inferior branch of the left middle cerebral artery (MCA) (Fig. 1B). Transthoracic and transesophageal echocardiography and 24-hour Holter tests were performed for the evaluation of the embolic source, but there were no abnormal findings. Agitated saline test with transcranial Doppler monitoring did not show any microembolic signals suggesting right-to-left shunt. He was discharged from the hospital, and the modified Rankin scale score at discharge was 0. We classified his stroke mechanism as embolic stroke of undetermined source (ESUS) and started antiplatelet treatment.
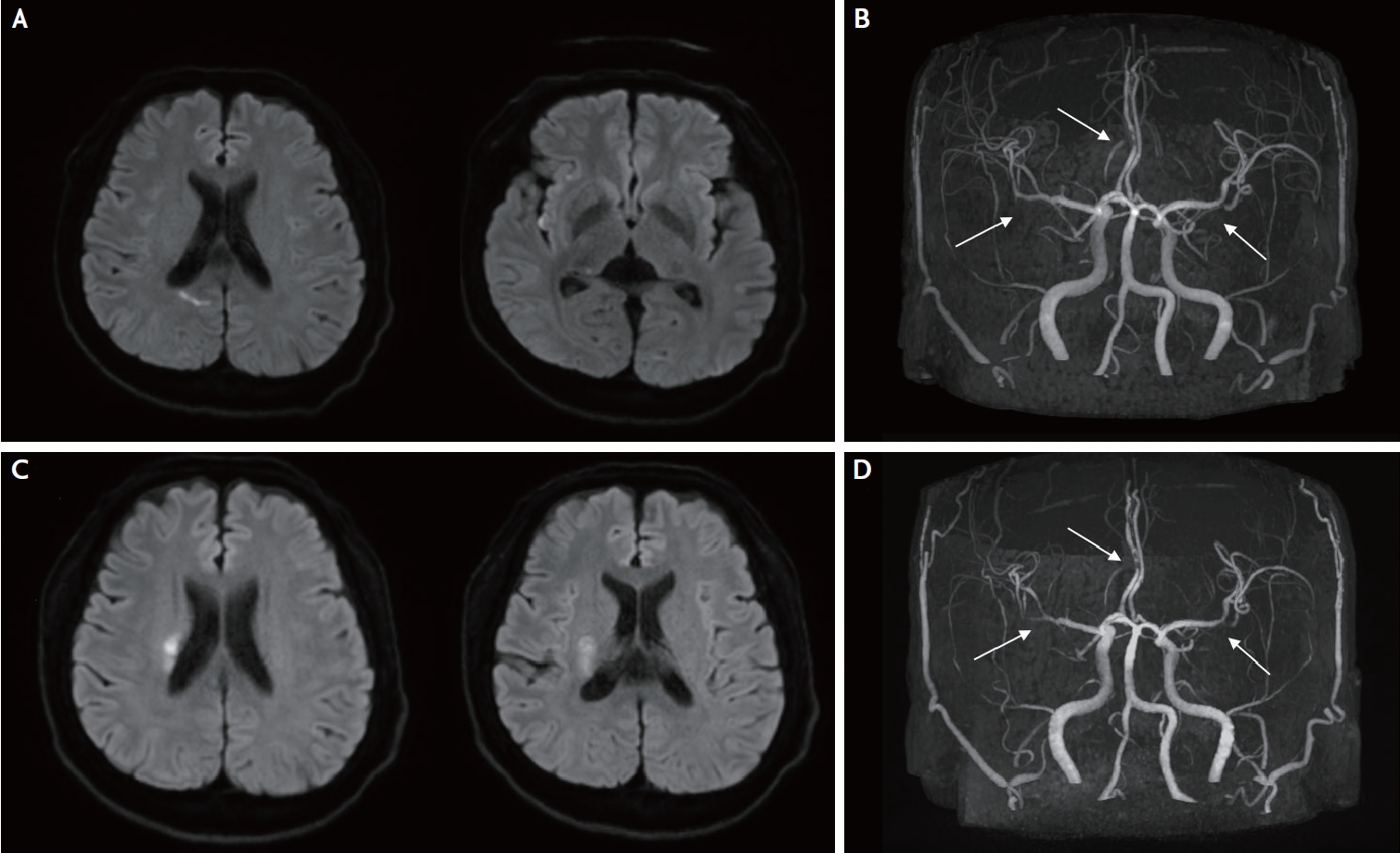
Brain magnetic resonance images and angiographies of the patient. Diffusion weighted image (DWI, A) and intracranial magnetic resonance angiography (MRA, B) of the patient at the first event. Diffusion restriction lesion is noted in the right insular cortex and parietal subcortex, and MRA shows stenosis in the A2 segment of the right anterial cerebral artery (arrow) and proximal portion of inferior branch of the left middle cerebral artery (MCA, arrow). Another DWI (C) from the stroke recurrence at 3 months after the first event shows diffusion restriction lesions at the right corona radiata. MRA (D) shows moderate progression of the right MCA (arrow) stenosis from prior imaging.
After 3 months, he visited our hospital again with recurrent left side weakness (Medical Research Council, MRC scale of upper and lower limbs, 4+/4+). There were diffusion restriction lesions in the right corona radiata and basal ganglia, as seen on DWI (Fig. 1C). The MRA revealed equivocal changes in the right ACA and left MCA stenosis, but moderate progression of the right MCA stenosis (Fig. 1D). Early neurological deterioration occurred, and left side weakness was fluctuating (MRC scale of upper and lower limbs, 3/3 to 4+/4+). The lowest National Institutes of Health Stroke Scale score was 8 within seven days after second stroke onset. VWI was performed to evaluate the arterial wall status, and there were multiple intracranial arterial stenoses and multifocal, segmental, concentric enhancement and thickening of the vessel walls suggestive of cerebral vasculitis (Fig. 2A-C). This was likely to have been caused by UC-associated cerebral vasculitis, and he was started on immunosuppressive therapy with methylprednisolone 500 mg/day for 5 days. His symptoms were stabilized and gradually improved. Three months after the onset of symptoms, follow-up VWI showed marked attenuation of enhancement in both MCAs and equivocal changes of diffuse wall thickening with plaque and wall enhancement of the right ACA (Fig. 2D-F).

Vessel wall imagings (VWIs) of the patient before and after immunosuppressive therapy. Sagittal PD VISTA precontrast sequence (A) shows diffuse wall thickening with plaque in right (A1) and left (A2) middle cerebral arteries (MCAs) (arrows), and sagittal (B) axial (C) T1 postcontrast VWIs show concentric enhancement in right (B1) and left (B2) MCAs (arrows). Six months after initial stroke, the VWIs (D-F) show marked improvement of wall thickening and enhancement in right (D1, E1) and left (D2, E2) MCAs (arrows).
DISCUSSION
The stroke classification of our patient was initially given as ESUS at the time of first cerebral infarction. However, the patient suffered a recurrent stroke despite antiplatelet treatment. Based on the patient’s history of UC, VWI technology was used to confirm the etiology of UC-associated vasculitis, which was treated by immunosuppressive therapy.
A meta-analysis of cohort studies of IBD patients revealed a statistically significant association between IBD and the risk of stroke (relative risk, 1.32; 95% confidence interval: 1.20–1.44).4 Thromboembolism occurring from a non-specific hypercoagulable state as well as cerebral vasculitis caused by an immune reaction are suggested as the possible pathomechanisms of cerebral infarction in UC patients.2 Several studies have documented that UC is highly associated with ischemic stroke and linked to cerebral vasculitis.5,6 VWI done in cerebral vasculitis cases show thickening of arterial walls and multifocal, concentric enhancement in the related vessels; therefore, VWI could prove helpful in confirming cerebral vasculitis.3,7 It is difficult to identify vascular stenosis at the distal portion of a time-of-flight MRA, in which case VWI can help in assessing the stage of the disease. Because it is sometimes difficult to completely distinguish atherosclerotic eccentric arterial wall thickening from vasculitic concentric vessel wall changes, the possibility of atherosclerotic stroke due to a vulnerable plaque limited to intracranial vessels cannot be completely ruled out. However, our patient had more homogeneous, smooth, and concentric arterial wall thickening with clear contrast enhancement in axial and sagittal VWIs, and the initial enhancement and wall thickening in our patient were markedly improved in 3 months from treatment. In addition, there were no proven evidences of atherosclerosis of coronary arteries, peripheral arteries, and extracranial arteries in our patient.
In this case, serial follow-up VWIs of cerebral vasculitis in a patient with UC were provided. VWI can be a useful tool to confirm not only the diagnosis of cerebral vasculitis, but also the therapeutic effect of the immunosuppressant.
Notes
Ethics statement
Kyung Hee University Hospital Institutional Review Board (IRB) exempted IRB approval because our single case report did not perform any prospective intervention. Written informed consent was obtained from the patient for publication of this case report.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Sources of Funding
None.
Conflicts of Interest
No potential conflicts of interest relevant to this article was reported.
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
