The Role of Transcranial Doppler in Patients with Cryptogenic Stroke
Article information
Abstract
Identifying the stroke mechanism is crucial for secondary prevention of ischemic stroke. However, approximately 20% to 30% of patients with ischemic stroke do not display a discernible cause after standard diagnostic evaluation and are classified as having cryptogenic stroke. Most cryptogenic strokes are believed to be of embolic origin, referred to as embolic stroke of undetermined source. Several potential causes, including occult atrial fibrillation, aortic arch atheroma, non-stenotic atherosclerosis, coagulopathy, and patent foramen ovale (PFO), are significant contributors to cryptogenic stroke. Among these, PFO-associated strokes often manifest as small cortical lesions in the posterior circulation and are associated with fewer traditional risk factors. Ischemic stroke is considered a high risk if there is a large PFO with significant shunting or the presence of an atrial septal aneurysm. Although transesophageal echocardiography is regarded as the gold standard for diagnosing PFO, its invasiveness, high cost, and challenges in certain stroke patients necessitate alternative approaches. Recently, transcranial Doppler (TCD) has emerged as a valuable screening tool for detecting high-risk PFOs, as supported by recent research. Accurate determination of the culprit PFO responsible for ischemic stroke remains crucial for guiding appropriate treatment decisions. This article provides a comprehensive review of the different modalities used to diagnose PFO, strategies for assessing the risk of PFO causing paradoxical embolism, and the role of TCD in patients with cryptogenic stroke caused by other etiologies.
INTRODUCTION
Ischemic stroke can occur due to various mechanisms, and identifying the underlying cause is crucial for determining the appropriate treatment. In cases where the exact mechanism cannot be determined (known as cryptogenic stroke), the risk of recurrent stroke is higher.1 It is estimated that approximately 20% to 30% of patients with ischemic stroke do not have a clear cause identified after a standard diagnostic workup, and cryptogenic strokes are particularly common among young patients.2
Most cryptogenic strokes are believed to be embolic in nature, originating from the proximal arteries, heart, or venous sources such as right-to-left shunt (RLS). Embolic stroke of undetermined source (ESUS) accounts for 80% to 90% of all cryptogenic strokes, and important contributing factors include occult atrial fibrillation (AF), aortic arch atheroma, non-stenotic atherosclerosis, coagulopathy, and patent foramen ovale (PFO).2,3 Given that the treatment strategy and prognosis can vary depending on the cause, early identification of the embolic source in cryptogenic stroke is crucial and necessitates a comprehensive diagnostic workup.
Transcranial Doppler (TCD) monitoring is a valuable tool for evaluating cryptogenic stroke, particularly in identifying PFO. TCD can detect microembolic signals (MESs), irregular cardiac rhythm, and the presence of RLS.4 However, it should be noted that PFO is found in approximately 20% to 25% of the general population, making it challenging to determine whether PFO is the exact cause of a stroke, even in cases of cryptogenic strokes. Therefore, it is important to differentiate between PFO-associated stroke (PFO-stroke) and cryptogenic stroke caused by other underlying etiologies, as well as to identify high-risk PFO cases. This review aimed to explore the role of TCD in the evaluation of cryptogenic stroke and to discuss the pathogenesis, diagnostic methods, and treatment approaches for patients with ESUS, with a specific focus on PFO-strokes.
ROLE OF TCD IN PFO-ASSOCIATED STROKE
1. Epidemiology and pathophysiology of PFO
PFO, a congenital heart defect characterized by a hole in the atrial septum, is the most common congenital heart disease. A large case-control study reported the prevalence of PFO increases by up to 55% in cryptogenic stroke.5 However, it is known that the prevalence of stroke gradually decreases with age, because the PFO gradually becomes occluded with age.6 On the other hand, the size of the remaining PFO increases with age.7 Strokes caused by PFO usually occur as paradoxical embolism. A paradoxical embolism is a specific type of embolism in which the embolus travels from the venous circulation to the systemic arterial circulation without passing through the pulmonary circulation. For paradoxical embolism to occur, there are several prerequisites: 1) thrombosis must occur in the vein; 2) a shunt in which venous and arterial blood are directly connected must be present; and 3) the pressure of venous blood must be higher than that of arterial blood.
A pressure reversal in which the right atrial pressure temporarily increases compared to that of the left atrium occurs during early diastole and during isovolumetric ventricular contraction during the normal cardiac cycle, but this is not a problem in the absence of PFO.8 If a PFO is present, the Valsalva maneuver induced by coughing or conditions such as pulmonary hypertension may increase right atrial pressure, leading to RLS and paradoxical embolism. However, lower-extremity venous thrombosis is not frequently found, and blood clotting disorders are not common in patients with PFO-stroke.9-12 Therefore, in addition to paradoxical embolism, other mechanisms such as ischemia caused by hypoxia due to venous blood inflow and saddle thrombus occurring within the PFO have been suggested as alternative mechanisms.13
2. Imaging and clinical features of PFO-associated stroke
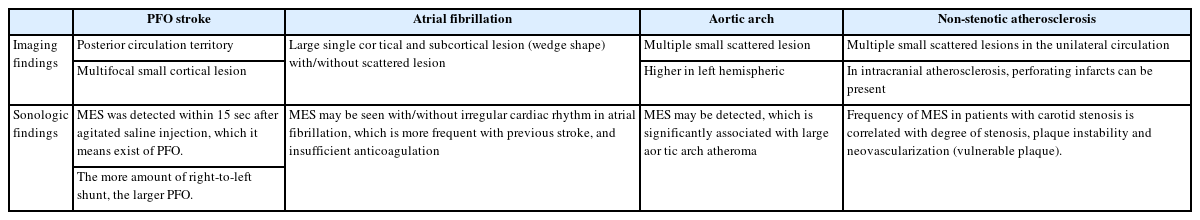
To cause paradoxical embolism, a thrombus must pass through the shunt. Therefore, in a PFO-stroke, small ischemic lesions are observed in the cerebral cortex because mostly small thrombi are embolized to the brain.14 For this reason, many patients show multiple lesions in the juxtacortical region on fluid-attenuated inversion recovery (FLAIR) imaging. The Valsalva maneuver not only increases RLS but also somewhat increases blood flow to the posterior circulatory system.15 Therefore, it is known that cerebral infarction caused by PFO during the Valsalva phase occurs frequently in the posterior circulation.14,16
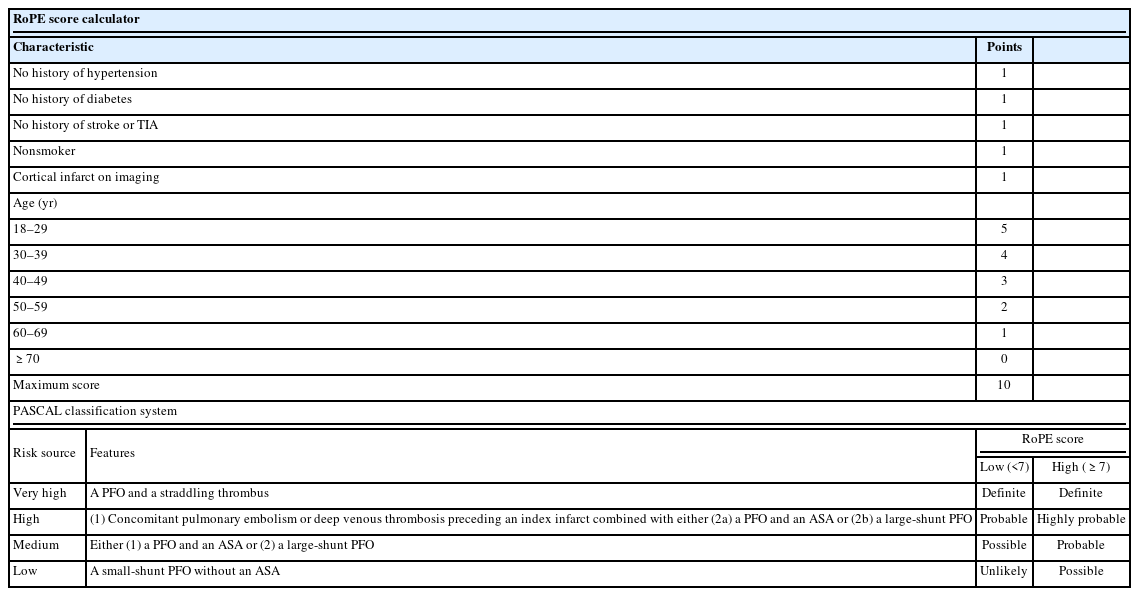
In general, patients with stroke due to atherosclerosis have risk factors such as old age, hypertension, diabetes, hyperlipidemia, smoking history, and history of stroke or transient ischemic attack (TIA). However, patients with PFO-stroke do not have many of these. Therefore, the Risk of Paradoxical Embolism (RoPE) score was developed considering these risk factors and neuroimaging findings. The RoPE score can predict the attributability of PFO to stroke (Table 1).17 The higher the score, the higher the probability the stroke is caused by PFO. Six points or higher is regarded as a high attributability of the stroke to PFO. On the other hand, the more likely PFO is to cause a stroke, the lower the risk of stroke recurrence.17 Although the RoPE score is helpful for quantitative evaluation, it has limitations in that it does not include anatomical findings such as the size of the RLS and the presence of atrial septal aneurysm (ASA), which are high-risk factors for cerebral infarction due to PFO. Therefore, it is necessary to confirm the presence of high-risk PFO using echocardiography.
3. Diagnosis of PFO
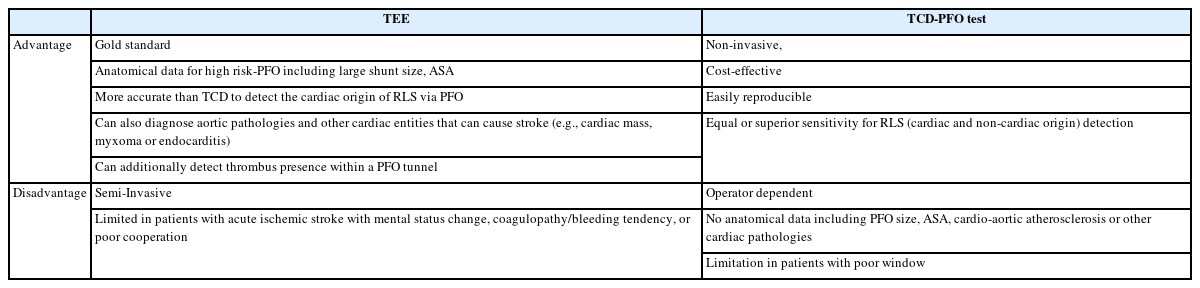
Transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), and transcranial Doppler (TCD) are helpful in confirming the diagnosis of PFO and RLS.18,19 Among these, TEE is considered the gold standard. However, in patients with acute stroke, performing TEE may be challenging because of decreased consciousness, coagulopathy, and difficulties in cooperation, particularly with efficient Valsalva maneuvers. In such cases, agitated saline TCD monitoring may offer an advantage in detecting RLS by revealing MESs. Although it is easier to perform the Valsalva maneuver with TCD than TEE, TCD does not provide anatomical information (Table 2).18,19
Recently, cardiac computed tomography angiography has also been used to define the heart’s anatomy.20 Nevertheless, TEE remains the most important test for diagnosing PFO-stroke and determining appropriate treatment. This is because TEE allows evaluation of the presence of ASA and the size of the foramen, which are crucial in determining whether or not it is a high-risk PFO.21
1) Procedure and interpretation of PFO tests
TEE is a procedure that utilizes an ultrasound tube with a probe inserted through the mouth and placed in the esophagus to examine the back of the heart using ultrasound. The procedure begins with the insertion of an intravenous catheter into the patient’s arm. A syringe with 1 cc of air and another syringe with 9 cc of normal saline are connected to both sides of a three-way cock. The mixture is agitated by back and forth movement between the syringes approximately 10 times to create air bubbles. During TEE, the right and left atria are visualized, and when agitated blood-tinged saline is intravenously injected, air bubbles first appear in the right atrium. If a bubble is observed in the left atrium within 3 to 5 cardiac cycles, it is indicative of an intracardiac RLS. A PFO larger than 2 mm or accompanied by an atrial septum is considered a high-risk PFO owing to its higher potential for inducing cerebral infarction.22-24
TCD involves monitoring and ultrasound assessment of both middle cerebral arteries (MCAs) with the patient in a supine position. Similar to TEE, MESs are recorded after the injection of agitated blood-tinged saline in the resting state. The Valsalva maneuver is then performed by reinjecting agitated saline and maintaining maximal inspiration while forcefully exhaling against a closed glottis for 10 seconds. After releasing the Valsalva maneuver and resuming normal breathing, MESs are recorded. MESs observed on TCD are more prominent in the right MCA than in the left MCA. However, in some cases, the temporal window may not provide clear visibility; in such situations, monitoring the vertebrobasilar artery can be considered an alternative.25
The number of MESs detected on TCD allows for the classification of RLS according to the International Consensus Criteria and Spencer grading system.26 Both grading scales exhibit high accuracy in identifying shunt presence. However, the Spencer grading system demonstrates a superior positive predictive value for detecting large and functional RLS compared with the International Consensus Criteria, along with a lower false-positive rate.27 Moreover, the timing of MES detection in TCD can help differentiate between intra- and extracardiac RLS. Typically, if MESs are observed within 15 seconds after agitated saline injection, it suggests an intracardiac shunt. Conversely, if MESs appear 15 seconds after saline injection, they are more likely to be caused by an extracardiac shunt, necessitating chest computed tomography (CT) to evaluate the presence of a pulmonary arteriovenous fistula. Some patients exhibit MESs even in the resting state before the Valsalva maneuver (persistent PFO), whereas others exhibit MESs only during the Valsalva maneuver (provoked PFO). Provoked PFO is associated with a higher incidence of posterior circulation infarction.25 As MESs observed on TCD align with the direction of blood flow, signals detected in the opposite direction are likely to be artifacts. TCD screening plays a significant role in identifying high-risk PFO in patients with suspected PFO-stroke. In fact, TCD is more sensitive but less specific than TTE for the detection of PFO in patients with cryptogenic stroke.28 Additionally, when TCD reveals an RLS with a Spencer grade of 3 or higher, there is a high likelihood of confirming a high-risk PFO through TEE (Fig. 1).29

Transcranial Doppler monitoring reveals the presence of multiple microembolic signals (MESs), corresponding to Spencer grade 3. In the Spencer scale, the right-to-left shunt and potential patent foramen ovale size are graded as follows: grade 1 (1–10 MESs), grade 2 (11–30 MESs), grade 3 (31–100 MESs), grade 4 (101–300 MESs), and grade 5 (>300 MESs).
4. Treatment of PFO-associated stroke
In cryptogenic stroke patients with PFO, the annual stroke recurrence rate is less than 2%, which is lower than that of other stroke etiologies. However, due to its occurrence primarily in young individuals, the cumulative incidence rate remains significant.30 The risk of stroke recurrence increases if the PFO size is larger than 2 mm and the Spencer grade is high, particularly when accompanied by ASA, which can result in a recurrence rate of up to 15%.31 Therefore, appropriate medication and treatment procedures are crucial.
The optimal medical treatment for PFO remains uncertain, and few studies have compared antiplatelet and anticoagulant agents. The NAVIGATE ESUS study comparing rivaroxaban and aspirin in patients with ESUS showed no superiority of rivaroxaban in preventing recurrent stroke and indicated a higher risk of bleeding.32 A subanalysis of patients with ESUS exhibiting PFO did not provide sufficient evidence to support a difference in recurrent ischemic stroke risk between rivaroxaban and aspirin. However, a meta-analysis combining data from multiple trials suggested favorable outcomes for anticoagulation therapy in terms of ischemic stroke risk reduction.33
Paradoxical embolism through the RLS is considered the primary cause of cerebral infarction in patients with PFO. Several observational studies demonstrated lower stroke recurrence rates after percutaneous PFO closure than after medical treatment alone. However, the past prospective randomized trials (CLOSURE I, RESPECT, PC) comparing PFO closure to medical treatment failed to demonstrate the superiority of the procedure.34-37 Inadequate patient selection, improper instrumentation, high procedure failure rates, and poor study design were identified as contributing factors to these failures.38 However, extended follow-up results of the RESPECT trial showed benefits from PFO closure, and recent randomized clinical trials (RCTs; CLOSE, Gore REDUCE, DEFENSE PFO) demonstrated significant reductions in recurrent stroke with PFO closure in selected patients aged ≤60 years with embolic-appearing ischemic stroke and RLS.39-42 The risk reduction was more pronounced in patients with high-risk PFO, such as ASA or large shunt.43 It is important to carefully select patients expected to benefit from PFO closure. Nonetheless, meta-analyses have consistently reported increased rates of newly detected atrial fibrillation in PFO closure groups compared with medical therapy groups.44,45
The PFO-associated Stroke Causal Likelihood (PASCAL) Classification System was developed to improve patient selection for PFO closure by incorporating anatomic and physiologic features alongside the RoPE score (Table 1). This system estimates the probability that stroke is associated with PFO in patients with embolic infarct topography without other major sources of ischemic stroke. It categorizes the likelihood of PFO-stroke as unlikely, possible, probable, highly probable, or definite, offering better differentiation of recurrent stroke risk compared to the RoPE score.46 The PASCAL system showed a reduction in ischemic stroke incidence with PFO closure in a study combining data from RCTs. The absolute risk reduction was dependent on the PASCAL category, and device-associated adverse events were higher in the unlikely group. For patients ≤60 years old with a possible, probable, or definite likelihood of PFO-stroke, device closure in addition to medical therapy is suggested.47 However, the possibility of the PFO being an “innocent bystander” and other mechanisms being responsible for the stroke should be considered, particularly in older patients with known stroke risk factors.
5. Unmet needs of PFO-stroke
Previous RCTs have not studied the relative benefit of PFO closure in patients with high-risk versus low-risk PFO for secondary prevention of cerebrovascular events in cryptogenic stroke patients.48 One study found an increased risk of stroke recurrence in patients with severe RLS compared to those with low-risk PFO.49 Subgroup analysis of the RESPECT trial showed greater benefit in stroke reduction for patients with high-risk PFO features, but no evidence for low-risk PFO closure currently exists.48 Additional large studies are needed to address this issue.
The effect of PFO closure in patients aged ≥60 years is not well studied, except for a few patients in the DEFENSE-PFO trial.50 Current guidelines recommend PFO closure for high-risk PFO in patients under 60 years of age, but the benefits for older patients and the age cut-off for potential benefits are unclear. Subgroup analysis of the DEFENCE-PFO study suggests that PFO closure may be effective in preventing recurrent stroke in patients aged ≥60 years.51 The ongoing COACH-ELDERLY ESUS trial (NCT05238610) aims to further evaluate the clinical effectiveness of PFO closure in patients over 60 years of age.
However, the optimal antithrombotic therapy duration after PFO closure remains unclear. Periprocedural antiplatelet therapy is typically administered for at least 6 months, followed by a variable duration of up to 5 years.52 A recent study compared short-duration (6 months) versus extended-duration (>6 months) antithrombotic therapy after PFO closure and found no impairment in clinical outcomes or increased risk of recurrent stroke at 10-year follow-up when antiplatelet therapy was discontinued 6 months after a successful procedure.53 However, this study included patients with low cardiovascular risk factors, and further research is needed to investigate this in the future.
THE ROLE OF TCD IN OTHER CRYPTOGENIC STROKES
Monitoring MESs using TCD can aid the detection of high-risk embolic sources and provide insights into stroke recurrence prediction. MESs are frequently observed during the acute phase of ischemic stroke, and bilateral detection may indicate cardiac embolism.54 MES frequency varies depending on the stroke mechanism. In patients with hemispheric stroke, MESs were detected in 20.5% of patients with large artery atherosclerosis, 17.1% with cardioembolism, and 5% with cryptogenic stroke, while they were absent in patients with small vessel occlusion.55 The presence of MESs has been associated with early recurrence and more severe stroke at presentation. A study revealed that 42% of MES-positive patients exhibited new ischemic lesions on follow-up diffusion-weighted magnetic resonance imaging (MRI), compared with only 15% of MES-negative patients, when monitored 48 hours after symptom onset.56
1. Non-stenotic atherosclerosis
Traditionally, ischemic strokes or TIAs were primarily attributed to carotid disease when the stenosis exceeded 50%, neglecting the potential of distal embolization in cases of mild stenosis (<50%).57 However, recent advancements in imaging have allowed for better assessment of plaque composition, revealing that even in mild stenosis, embolism can occur through plaque rupture or platelet activation. Plaque characteristics, such as intraplaque hemorrhage, lipid-rich necrotic core, and fibrous cap thinning or rupture, are associated with an increased risk of embolic events. Studies using CT angiography and high-resolution MRI have demonstrated that nonstenotic carotid and intracranial plaques can serve as potential sources of embolism in patients with ESUS.58,59 TCD monitoring is valuable for evaluating nonstenotic atherosclerosis as a potential cause of ESUS. MES occurrence in patients with carotid stenosis correlates with the degree of stenosis, and in individuals with symptomatic carotid artery disease, MES occurrence is linked to plaque instability and neovascularization. 60,61 Thus, MESs detected through TCD monitoring in patients with nonstenotic atherosclerosis suggests the possibility of an unstable plaque and a potential embolic source. Multiple lesions in the unilateral circulation or scattered lesions within one vascular territory are indicative of large artery atherosclerosis, whereas intracranial atherosclerosis can lead to perforating infarcts (Table 3).25
2. Aortic arch atheroma
Aortic arch atheroma increases the risk of ischemic stroke, particularly in the elderly, owing to its potential for embolization. Aortic arch embolism has a high tendency to cause left hemispheric stroke and multiple small infarcts.25 TEE or CT is commonly used to assess atheroma. The highest risk is attributed to a proximal complex plaque, defined as ≥4 mm thick, ulcerated, or containing mobile thrombi, given its proximity to the main neck vessels.62,63 However, recent evidence suggests that plaque located more distally in the descending aorta may also pose a risk. During diastole, significant retrograde flow occurs, potentially allowing thrombi to reach the neck vessels and ultimately result in stroke.62 Studies have demonstrated a significant association between TCD-detected MESs and large aortic arch atheroma in elderly patients with acute ischemic stroke, highlighting the usefulness of TCD in assessing the embolic risk of aortic atheroma.64
3. Occult atrial fibrillation
Occult AF is estimated to be the underlying cause of cryptogenic stroke in up to 30% of cases. This type of AF is typically asymptomatic and intermittent, posing challenges for its detection. However, identifying this arrhythmia is crucial because anticoagulation therapy significantly improves secondary stroke prevention.65 Cardiac monitoring plays a key role in detecting AF, and in patients with cryptogenic stroke using implantable cardiac monitors, AF was found in 40% of cases, with an average detection time of 8 months. TCD monitoring can also be used to identify MESs in patients with AF. The prevalence of AF and MESs varies between 13% and 30% across different studies, and their detection is more common in patients with a history of stroke, inadequate anticoagulation, or increased platelet aggregation.66,67 However, asymptomatic patients with solely AF rarely exhibit MESs on TCD monitoring.68,69 Therefore, TCD monitoring holds promise for identifying individuals at high risk of embolism associated with AF. Notably, the prompt initiation of anticoagulation with non-vitamin K antagonist oral anticoagulants can lead to the disappearance of MES. When patients present with multiple lesions involving different territories, or a single large cortical and subcortical lesion on MRI, occult AF should be suspected.
CONCLUSION
Cryptogenic stroke is a common occurrence that typically affects younger patients with fewer traditional stroke risk factors. Among potential causes, PFO is significant, although it has also been observed in the general population. Therefore, careful evaluation is necessary to determine whether PFO is the underlying cause of stroke. TCD has demonstrated reliability in detecting PFO in patients at high risk for cryptogenic stroke who could benefit from device closure. In terms of feasibility, TCD may be more suitable than TEE, which is considered semi-invasive, especially in patients with acute stroke. Furthermore, TCD monitoring for MESs is a non-invasive and cost-effective bedside test that can provide additional information to identify the embolic source or evaluate its activity. However, owing to its time-consuming nature, the clinical use of MES detection is somewhat limited. Therefore, it is reasonable to selectively employ TCD monitoring when necessary.
Notes
Ethics Statement
Institutional Review Board approval and patient consent were not necessary because this is a review article.
Availability of Data and Material
The data supporting the findings of this study are available from the corresponding author (Pf. Bum Joon Kim) on request.
Sources of Funding
This research was supported by the Brain Convergence Research Program of the National Research Foundation (NRF), funded by the Korean government (MSIT) (No. 2020M3E5D2A01084576), and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1A2C2100077).
Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Acknowledgements
None.