Occipital Steal Syndrome with Carotid Artery Occlusion–A Physiologic Study with Transcranial Doppler
Article information
Abstract
Occipital or posterior steal syndrome is a constellation of signs and symptoms that arise from abnormal shunting of blood flow from the posterior cerebral circulation into its anterior counterpart leading to transient episodes of neurological deficits due to posterior circulation ischemia. A 45-year-old woman who experienced transient visual phenomena during breath holding was evaluated by transcranial Doppler sonography (TCD) for changes in cerebral blood flow. TCD revealed that the anterior cerebral vascular system was shunting blood away from its posterior counterpart in the setting of hypercapnia, resulting in visual symptoms. Using permissive blood pressure control, the steal phenomenon was resolved.
The main function of the posterior communicating (pComm) artery relates to its role in connecting the anterior and posterior cerebral circulations. In cases when either the basilar or internal carotid arteries are occluded, the posterior communicating artery provides the brain with a viable blood supply by conducting blood from the contralateral circulation.1 Occipital or posterior steal syndrome is a constellation of signs and symptoms that arise from abnormal shunting of blood flow from the posterior cerebral circulation into its anterior counterpart and is characterized by transient episodes of neurological deficits due to posterior circulation ischemia.2
CASE
A 45-year-old female with significant cardiovascular history including multiple myocardial infarctions (due to both coronary artery thrombosis and dissection), ischemic heart failure requiring heart transplant with inconclusive pathology (post-transplant ejection fraction Normal), being treated with strict blood pressure control was referred to the Mount Sinai Hospital Neurosonology lab for further evaluation after experiencing six months of static bright lights only with exertion. Neuro-ophthalmology evaluation was unremarkable, as was her physical and neurological exam. There was no evidence of an infarct or hemorrhage on neuroimaging. Vessel imaging including the computed tomography angiography of her head and neck and MR angiography of her head revealed the left anterior cerebral artery (ACA) having a smaller caliber than the right, with stenosis at its origin. In addition, the pComm artery on the left is well formed, while the internal carotid artery (ICA) on the left is completely occluded (Fig. 1).
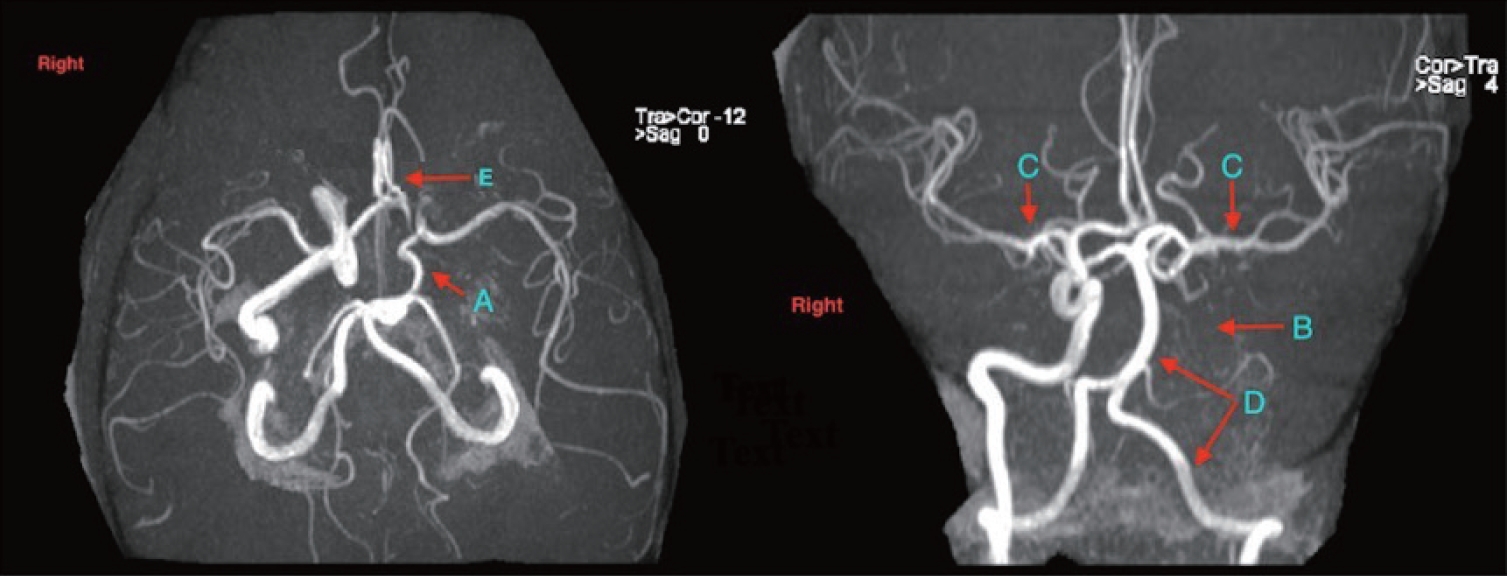
Magnetic resonance angiography of the head demonstrating. (A) Well-formed posterior communicating artery. (B) Occluded left internal carotid artery. (C) Middle cerebral artery, normal in caliber. (D) Posterior circulation (including the vertebral and basilar arteries). (E) Stenosis at the left anterior cerebral Artery origin.
Transcranial Doppler (TCD) Findings
Initial TCD demonstrated normal flow velocity with reduced intracranial pulsatility of the left middle cerebral artery (MCA), without collateral circulation from the anterior communicating artery. Collateral circulation was provided by the basilar artery and the left posterior cerebral artery in high volume (Fig. 2A).
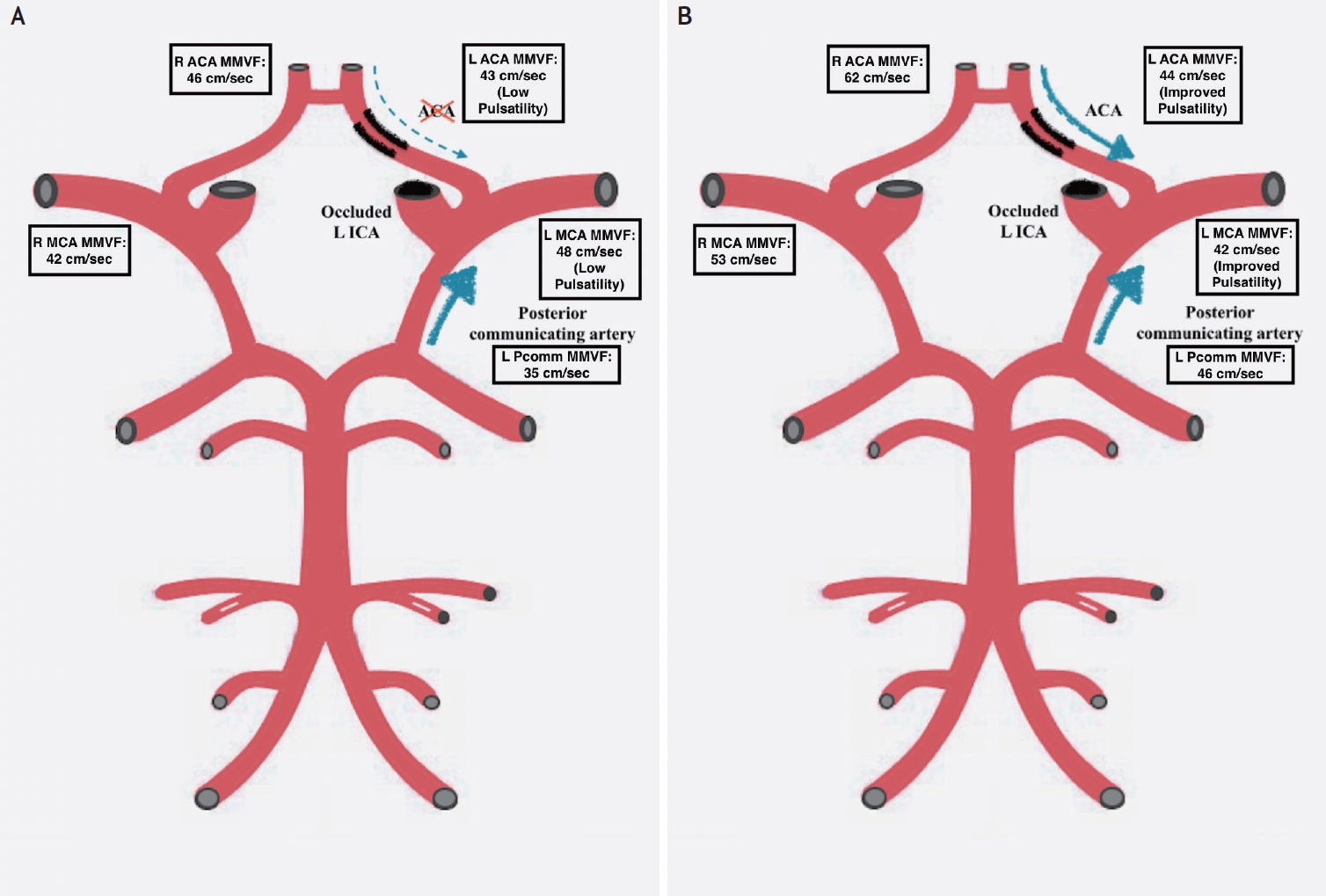
TCD findings. (A) Initial TCD: normal flow velocity of the left middle cerebral artery, with collateral flow being provided by the basilar artery and left posterior cerebral artery in high volume (no collateral flow from aComm). (B) Repeat TCD at 3 months demonstrating an increase in blood pressure leading to an improvement in collateral flow. TCD, transcranial Doppler; MMFV, maximum mean flow velocity; aComm, anterior communicating artery.
The Breath Holding (BH) test was performed to assess for cerebrovascular reactivity. The purpose of the BH test is to induce a temporary increase in CO2 leading to cerebral vasodilation and thus an increase in mean blood flow velocity. In a healthy patient with intact vasomotor activity, the mean blood flow velocity is expected to increase steadily with the duration of breath-holding. As vessels are already dilated to compensate for poor collaterals, large vessel diseases are associated with the lowest dilatory reserve capacity and the absence of increased flow when the BH test is conducted. The breath-holding index (BHI) is a useful measure to assess for cerebrovascular reactivity. The BHI is calculated from the mean flow velocities of the MCA using TCD.3,4
After 30 seconds of the patient holding her breath, the mean flow velocity of the left MCA was measured. There was poor vascular reserve of the left MCA based on a breath holding index of 0.22 compared to a normal range of at least 0.78 (Fig. 3A). At the end of the breath-holding period, the patient experienced visual bright spots in both eyes, indicating relative ischemia in the occipital lobe when the collateral supply to the left cerebral hemisphere is under stress.
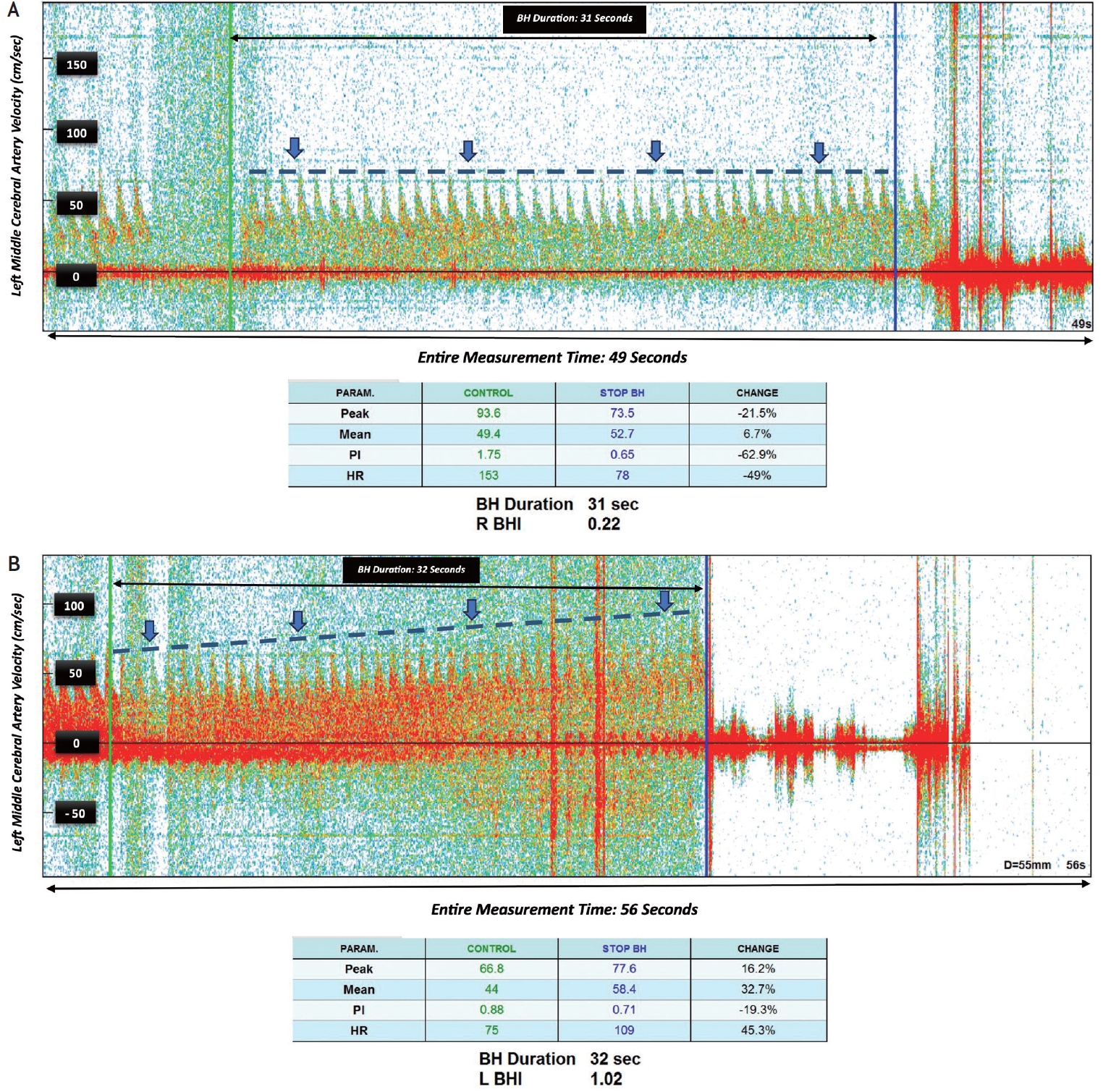
(A) After 30 seconds of the patient holding her breath, the velocity of left middle cerebral artery remained almost unchanged (blue line), with poor vascular reserve of the left middle cerebral artery represented by a BHI of 0.22 (normal 0.78). (B) Repeat TCD at 3 months demonstrating a BHI 1.02. During breath-holding, the mean blood flow velocity increases as time passes, as is expected for healthy individuals. TCD, transcranial Doppler; BH, breath holding; BHI, breath holding index.
The patient’s blood pressure up to this point had been strictly controlled to less than 120/80 due to underlying cardiovascular and renal disease. Considering her left ICA occlusion and stenosis of her left ACA, we conclude that the observed phenomenon was caused by limited collateral circulation from the right to left sides. As a consequence, blood was ‘stolen’ from the posterior circulation to the anterior circulation while at a reduced blood pressure, resulting in visual disturbances. We recommend liberalization of blood pressure to a moderate 140/90 range to assess for symptom resolution.
At a follow up visit three months after, the patient underwent a second TCD. The patient reported maintaining her blood pressure around 140/70 without a recurrence of visual symptoms, despite exertion. Compared to the prior study, the BHI increased from 0.22 to 1.02 and the patient did not develop symptoms during the visit, indicating good cerebrovascular reserve due to adequate collateral circulation (Figs. 2B, 3B). The specifics of the transcranial Doppler study are outlined in the Supplementary Table 1.
DISCUSSION
Embryologically, the posterior communicating artery is considered part of the anterior circulation. It plays a significant role in the formation of the Circle of Willis by establishing connections between the internal carotid arterial system and the vertebro-basilar arterial system.1
Occipital or posterior steal refers to the abnormal redirection of blood flow from the posterior to the anterior circulation, leading to transient neurologic deficits. This phenomenon has been documented in two previous case reports involving a 17-year-old man with Meniere’s-like symptoms and a 47-year-old woman with episodic headache and loss of consciousness. In this report, we present a unique case of a patient experiencing transient visual phenomena during the breath-holding test, with evidence of blood flow reversal between the anterior and posterior circulations on transcranial Doppler.2,5
Increasing the blood pressure in this patient improved collateral flow from the right anterior circulation to the left side (Fig. 2B) A more liberal blood pressure control was used to treat the patient’s visual symptoms and limit the steal phenomenon; further treatment strategies have not been investigated to our knowledge, but could be conceived as more cases emerge. TCD is a helpful tool to study hemodynamic change in vaso-occlusive disease and improve medical management.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.31728/jnn.2023.00146.
Transcranial Doppler findings before and after changing blood pressure goal from below 120/70 to around 140/90
Notes
Ethics Statement
Informed consent is obtained and already submitted to the Journal. IRB approval is not applicable per our institution policy.
Availability of Data and Material
All data related to this study are included in the main text.
Sources of Funding
None.
Conflicts of Interest
No potential conflicts of interest relevant to this article was reported.
Acknowledgements
None.
