Middle Cerebral Artery Pulsatility Is Highly Associated with Systemic Blood Viscosity in Acute Ischemic Stroke within 24 Hours of Symptom Onset
Article information
Abstract
Background
Though blood viscosity (BV) is the major factor influencing pulsatility index (PI), it is unknown whether PI is related with BV in acute ischemic stroke. We explored the association between transcranial Doppler (TCD) PI and BV in acute ischemic stroke patient.
Methods
Between January 2018 and December 2018, patients aged 40 years or older with an ischemic stroke or transient ischemic attack within 24 hours of symptom onset were recruited for the study. The scanning capillary-tube viscometer was used to assess whole blood viscosity. Doppler signals showing the highest mean flow velocity were used for PI calculation.
Results
A total of 125 patients were enrolled for the study. The most frequent stroke subtype was lacunar stroke (42%), followed by stroke of undetermined etiology negative work-up (29%), and large artery atherosclerosis (19%). The mean middle cerebral artery (mMCA) PI was significantly associated with BV. Age, diabetes and BV were associated with mMCA PI, suggesting that increased mMCA PI is associated with old age, presence of diabetes, and increased BV.
Conclusion
Pulsatility of MCA is highly associated with BV in acute ischemic stroke patient within 24 hours of symptom onset. Our findings indicate that TCD PI may be a useful non-invasive imaging modality for exploring the viscosity of cerebral vessels, which in turn is related to systemic BV.
INTRODUCTION
Blood viscosity (BV) is the measurement of resistance of blood to flow and is an important rheological factor.1-3 Characterized by blood thickness and stickiness, BV is associated with elevated concentrations of blood cells and plasma components, and low deformability and high aggregability of erythrocytes.3,4 BV plays an essential role in the occurrence of vascular disease. High BV increases thromboembolic risk and is related with cerebro-cardiovascular disease.2,3,5 Several studies have demonstrated associations between BV and ischemic stroke.1,2,6 BV measurements are reportedly higher in lacunar stroke than in other stroke subtypes. Increased BV may impair microvascular tissue perfusion and be related to the pathomechanisms in lacunar stroke.2
The pulsatility index (PI) measures vascular resistance and exemplifies the shape of a spectral waveform.7 High-resistance transcranial Doppler (TCD) PI shows downstream vascular resistance in cerebral circulation. The most important factors affecting PI are the cerebral flow velocity and BV.8 Recently, the potential utility of the scanning capillary-tube viscometer (SCTV) to measure whole blood viscosity (WBV) has been highlighted in clinical application due to no requirements of periodic calibration or manual cleaning.9
Though BV is the major parameter influencing PI, it is unknown whether PI is related with BV in acute ischemic stroke. We hypothesized that PI would reflect the viscosity of cerebral vessels which, in turn, is related to systemic BV. Therefore, we explored the association between TCD PI and BV in acute ischemic stroke patient using SCTV.
SUBJECTS AND METHODS
1. Patients
Between January 2018 and December 2018, patients aged 40 years or older with an ischemic stroke or transient ischemic attack (TIA) within 24 hours of symptom onset were recruited for the study. For TIA, weakness or speech disturbance, dysarthria or dysphasia, had to be part of the symptom complex for greater than 5 minutes for patients to be eligible.10 The patients’ baseline demographics, vascular risk factors, and medical history were assessed at admission. Physical examination and systemic investigations were performed in all patients. Each patient underwent brain magnetic resonance imaging and at least one vascular imaging study, such as conventional angiography, MR angiography, or CT angiography. Echocardiography and 24-hour Holter monitoring were carried out in selected patients to detect the cardioembolic source. Stroke subtype classification was done according to the Trial of ORG 10172 in the Acute Stroke Treatment classification system.11 For the study, cardioembolism (CE) as a stroke subtype, or stroke of other determined etiology, were excluded. The study was approved by the Institutional Review Board of the Sanggye Paik Hospital (SGPAIK 2018-01-002). All patients provided written informed consent before study enrollment.
2. BV
The SCTV was used to assess WBV. The SCTV assesses systolic whole blood viscosity (SBV) and diastolic whole blood viscosity (DBV), which characterize viscosities at high and low shear rates, respectively. In this study, the WBV measured at a shear rate of 300 s−1 was selected as the SBV and a shear rate of 1 s−1 as the DBV.2 All blood samples were obtained before hydration therapy. Three mL of whole blood was drawn in ethylenediaminetetraacetic acid anticoagulant tube and all measurements were taken within 24 hours.
3. TCD
Methods for the use of TCD in our study have been published previously.7,12 Briefly, TCD was carried out with a Companion III (Nicolet Biomedical, Inc., Madison, WI, USA) according to the standard operating manual at 7 (±2) days after stroke onset. Doppler signals from the middle cerebral artery (MCA) were obtained at depths of 56, 58, and 60 mm. Doppler signals from the basilar artery (BA) were acquired at depths of 78, 80, and 82 mm. Gosling’s PI was calculated as the difference between the peak systolic and end-diastolic velocities divided by the mean flow velocity (mFV) in each artery. The depth showing the highest mFV were used as parameters for PI analysis. The TCD PIs in patients with either significant (>50%) stenosis or occlusion of MCA or BA were excluded for the study. If data were available for both MCAs, the mean MCA (mMCA) PI was used.
4. Statistical analysis
The vascular risk factors and laboratory findings were analyzed among groups using a one-way analysis of variance. Categorical data were examined by χ2 statistics. Multivariate linear regression analysis was carried out to examine the association of significant univariate variables. Statistical analyses were performed using SPSS version 25.0 for Windows (IBM, Armonk, NY, USA).
RESULTS
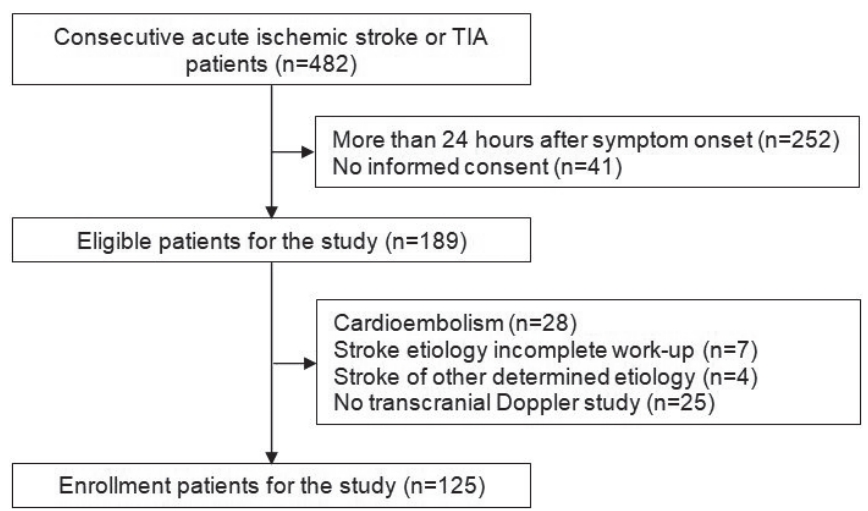
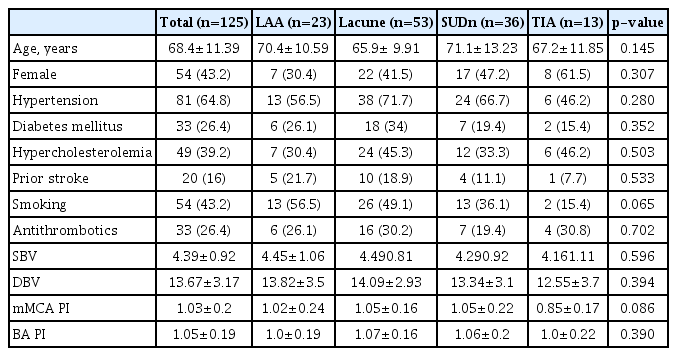
Fig. 1 shows the patient flow and identified reasons for exclusion from the study. In total, 189 patients within 24 hours of symptom onset were screened during the trial. Of these, 125 patients (66%) were enrolled for the study. The most frequent stroke subtype was lacunar stroke (53, 42%), followed by stroke of undetermined etiology negative work-up (36, 29%), and large artery atherosclerosis (23, 19%). Thirteen patients (10%) had TIA. The baseline characteristics are shown in Table 1 and 2. The mean age was 68.4±11.39 years, and 43% were women. Of these, 65% had a history of hypertension, 27% diabetes, 39% hypercholesterolemia, 20% stroke, and 43% were current smokers. There were no differences in baseline characteristics among stroke subtypes. No significant differences were observed in age, past medical history, and laboratory findings among groups. The mMCA and BA PI were also not different among groups (Table 1).
Table 3 shows the univariate linear regression analysis of the baseline characteristics in relation to mMCA and BA PI. PI was significantly associated with age, diabetes, hemoglobin, hematocrit, and BV (SBV and DBV). Table 4 demonstrates the multivariate linear regression analysis of PI dependent on BV. While there was no significant relationship between BA PI and BV, mMCA PI was significantly associated with BV (SBV and DBV). Age, diabetes and BV were associated with mMCA PI, suggesting that increased mMCA PI is associated with old age, presence of diabetes, and increased SBV and DBV.
DISCUSSION
PI is the reflection of multifactorial, pleiotropic events occurring in the systemic vascular system and becomes elevated with old age, presence of diabetes and hypertension, and cerebral small vessel disease (SVD).12,13 In this study, we explored the association between TCD PI and BV in acute ischemic stroke patient. Our study revealed that TCD PI was clearly associated with BV. Increased mMCA PI was associated with old age, presence of diabetes and increased SBV and DBV in acute ischemic stroke patient within 24 hours of symptom onset.
Age shows the most marked impact on PI. It has been well known that blood flow velocity is inversely related with age and PI is higher in the elderly.13 The higher hematocrit is associated with the lower the velocity and the PI by TCD generally.14 Univariate analysis demonstrated that PI was inversely related with hemoglobin and hematocrit in this study. These associations, however, were more remarkable in non-vascular neurological disorders than in patients with recent ischemic stroke, in concordance with multivariate analysis results.14 Regarding to diabetes, several studies demonstrate diabetes-related cerebral hemodynamic changes and suggest that the TCD PI reflects microangiopathic changes in cerebral vessels.15,16 Increased PI and reduced cerebrovascular reactivity of MCA is observed in diabetic patients and is closely related to the duration of diabetes.15 TCD PI and BV share common pathophysiological mechanisms. The most important effect of BV is in the microcirculation, where it contributes extensively to peripheral resistance.3 Upon reaching the arterioles, red blood cell (RBC) aggregates are dispersed due to increased shear after which RBCs flow as individual cells through the capillaries. After capillary passage, they reform aggregates within the collecting venules. Factors that increase RBC aggregation elevate flow resistance in the capillary system, which can be one of the major factors inducing increased PI.3
In our study, mMCA and BA PI were similar among stroke subtypes. One older study demonstrates that CE patients have the highest BV.17 Recent studies, however, suggest that BV is significantly higher in lacunar stroke.1,2 BV is dependent upon the aggregation of RBCs in small vessels at a low shear rate, equivalent to DBV.18 Since DBV has a greater impact on the tissue perfusion of small vessels than SBV, DBV can be more likely to influence cerebral SVD. When the blood passes the small vessels, an elevated DBV can exacerbate the flow disturbance and cause endothelial remodeling and luminal occlusion.2 Though there were no plausible explanations for these discrepancies among the studies, differences may exist in patient conditions. Firstly, dehydration may be associated with increased BV. One study demonstrates that BV at admission was significantly higher in lacunar stroke but not after 2 weeks of normal hydration.1 Secondly, results may be related to the different inclusion criteria of each study. In our study, patients with an ischemic stroke or TIA within 24 hours of symptom onset were enrolled. Informative studies regarding BV and acute ischemic stroke are limited and previous studies have not performed on this acute period.1,2,6 Further studies focusing on this acute period are required to assess the influence of BV in ischemic stroke. In contrast to mMCA PI, there was no significant relationship between BA PI and BV in this study. The cerebral vessels have rich adrenergic innervation which regulates vascular tone in response to various stimulations.19 Vessels in the posterior cerebral circulation may have a restricted vasodilatory response since they have fewer adrenergic neurons than in the anterior cerebral circulation.15 This can be attributed to the differences of mMCA and BA PI changes regarding to BV.
Our study had several limitations. The most important limitation was that it was a small cross-sectional and observational study. Hence, there may be hidden confounders. We were unable to examine plasma components such as fibrinogen or CRP. BV and PI were measured at a different time in this study. BV is a uniform entity over a short period of time and TCD PI is influenced by several physiological factors, such as the partial pressures of oxygen and carbon dioxide, and arterial pressure. Informative studies regarding TCD PIs and acute cerebral infarction are limited but our previous study shows that MCA and BA PIs are decreased at least for the first 14 days after the event regardless of study medication.8 We presumed that scheduled PI measurement was acceptable for the study. BV can rise with high levels of aggregating proteins and can be also modified by drug therapy such as anti-hypertensive drugs or statin.20 In this study, we did not assess the relationship between BV and medication. This trial enrolled only Korean patients, limiting the generalization of data to other geographic regions. These limitations should be considered when interpreting our data.
In conclusion, pulsatility of MCA is highly associated with BV in acute ischemic stroke patient within 24 hours of symptom onset. Our findings indicate that TCD PI may be a useful non-invasive imaging modality for exploring the viscosity of cerebral vessels, which in turn is related to systemic BV.
Notes
Conflicts of Interest
No potential conflicts of interest relevant to this article was reported.