Spinal cord infarction (SCI) is a disease with a low prevalence rate accounting for 0.3–1.0% of all strokes. SCI should be considered a cause for various clinical symptoms and signs such as motor and sensory symptoms, respiratory depression, and autonomic dysfunction.1 The causes of SCI vary widely. Above all, the common causes are aortic disease with traumatic or iatrogenic etiology and thrombotic occlusion.2 When compared to open transabdominal aortic aneurysm repair procedures, the SCI occurrence rate showed no significant difference in thoracic endovascular aortic repair (TEVAR). However, the occurrence of delayed onset SCI was greater in TEVAR than in open thoracoabdominal aortic aneurysm repair.3 Postoperative SCI is an important prognostic factor. The mortality rate is three times higher in patients with SCI than in those without SCI.3
Therefore, it is very important for surgeons and neurologists to keep in mind that SCI may arise from complications of aortic repair surgery or procedure. Here, we present a case of long segment SCI as a TEVAR complication.
CASE
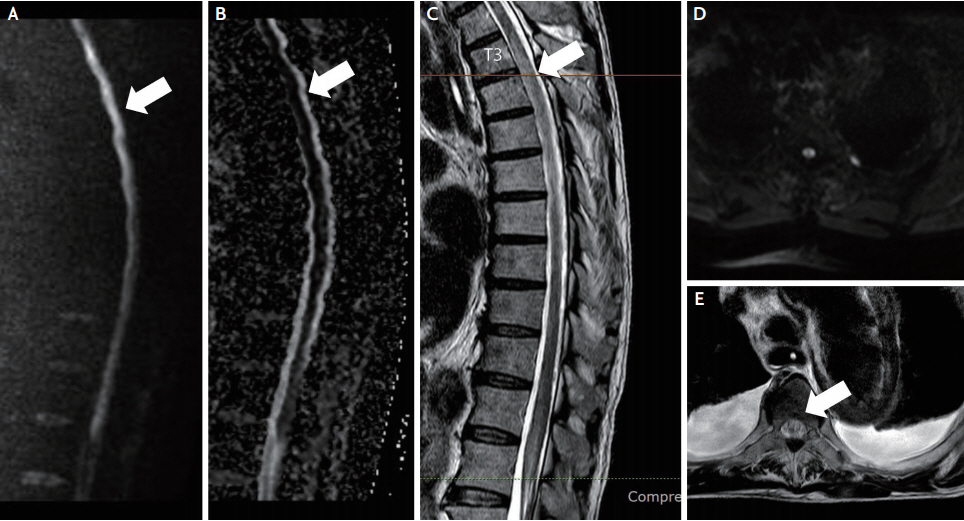
A 74-year-old man presented to the emergency department with chest pain that seemed to tighten both sides and radiated pain that stretched over his shoulder, which started 2 days before the hospitalization. He was on aspirin and cilostazol for three-vessel disease of the coronary artery. The patient had been previously diagnosed with atrial fibrillation, hypertension, and benign prostate hypertrophy. On coronary angiography, there was up to 90% stenosis with an ulcerative plaque in the left anterior descending coronary artery. Diffuse aortic aneurysm was found on computed tomography (CT) angiography of the thoracic and abdominal aorta. The aortic aneurysm extended from the ascending aorta, aortic arch, and descending thoracic aorta (Fig. 1) with a mural thrombus and a maximum diameter of 6.3 cm in the aortic arch, which is an indication for hybrid TEVAR.4 Therefore, elective surgery for thoracic aortic aneurysm was scheduled. The patient subsequently underwent on-pump beating-heart coronary artery bypass grafting and modified type II hybrid arch repair. The duration of the surgery was 13 hours 35 minutes in total, and mean blood pressure was maintained above 60 mmHg with invasive arterial blood pressure. The patient was unconscious until the second day after surgery and underwent brain CT, which showed no apparent lesion. The patient’s consciousness improved on the fourth day after surgery, but he was referred to a neurologist for paraplegia. On neurological examination, his consciousness was intact, but the patient was intubated, and his lower extremities showed more severe weakness (Medical Research Council [MRC] grade 0 on both sides) than the upper extremity (MRC grade 4 on both sides). Spine magnetic resonance imaging performed to rule out SCI revealed a wide range of restricted diffusion in the spinal cord from the thoracic vertebrae 1 level to the conus medullaris with T2 hyperintensity (Fig. 2). Dual antiplatelet (aspirin 100 mg daily, clopidogrel 75 mg daily) therapy was administered as secondary prevention. The patient was in the process of ventilator weaning, but on the fifth day after the surgery, failure of weaning and carbon dioxide (CO2) retention (partial pressure of CO2 ranging from 43.3 to 48.3 mmHg) were observed. At the same time, weakness of both upper extremities progressed from MRC grade 4 to MRC grade 2 on both sides. The possibility of a recurrent embolic infarction was considered at the level of the cervical cord and brainstem, but additional brain and spinal cord imaging were unavailable due to his critical condition. In addition, a CT scan of the abdomen and pelvis was performed due to elevation of the liver enzymes on the 4th day after the surgery and showed the perfusion delay on segment three and segment six tips of the liver and spleen. Discoloration and delayed capillary refill time in both feet led to the diagnosis of blue toe syndrome on the second day after the surgery.
However, anticoagulation therapy was delayed due to the patient’s poor bleeding control. The second surgery to explore and control the bleeding focus was performed 7 hours after the first surgery, in which no arterial bleeding was found. The hemoglobin level in complete blood count was 11.4 g/dL initially but dropped to 8.9 g/dL on the second day after the surgery.
Therefore, an additional 1 mg/kg enoxaparin daily, low molecular weight heparin after stabilization with massive transfusion of packed red blood cells, fresh frozen plasma, platelet cells, and cryoprecipitate were administered. Afterward, acute respiratory distress syndrome and acute kidney injury aggravated; thus, continuous renal replacement therapy was performed. However, the patient did not recover and died due to the deterioration of metabolic acidosis and low blood pressure.
DISCUSSION
If a patient develops sudden back pain with tightness, symmetric flaccid weakness, and sensory loss, the neurologist should not overlook the possibility of SCI. These symptoms are common in SCI. If a patient has undergone aortic repair surgery or procedure recently, the possibility of SCI increases. Previously, the popular opinion was that damage to the artery of Adamkiewicz is the main cause of SCI during aortic aneurysm repair.5 Therefore, clinicians aimed to avoid injury of the left vertebral artery or internal iliac artery.2 However, a recent concept is that hypoperfusion and insufficient compensation from the collateral flow are two main contributors to SCI.6 Supporting that concept, previous studies showed that the number of segmental arteries (i.e., intercostal and lumbar arteries) sacrificed and hemodynamic instability are important risk factors for SCI. Recent techniques to reduce the risk of SCI (i.e., multistaged procedure,6 perioperative cerebrospinal fluid drainage,7 hypothermia during proximal artery clamping,8 embolization, and intraoperative neurophysiologic monitoring9) are based on these laboratory and clinical studies. Some of these techniques have led to positive results.6,7,10
The pathogenesis of SCI after TEVAR seems multifactorial. The hypothesis is that atheroembolism of aortic plaque material into the segmental arteries supplying the spinal cord can also lead to SCI.2 In this patient, the evidence of postoperative atheroembolism was documented with blue toe syndrome and delayed arterial perfusion of the spleen, liver, and kidney in the abdominal and pelvic CT. Therefore, we can infer that mural thrombi embolization through the collateral network of blood vessels in the spinal cord, including the anterior spinal artery, contributed to the occurrence of SCI during and after TEVAR.
To a large extent, coverage of ≥2 vascular territories and coverage including the left subclavian artery are known surgical risk factors for SCI after TEVAR,5 which also explains this case. This case is a good example of long segment SCI with mural thrombi embolization after TEVAR.