Diabetic muscle ischemia or diabetic myonecrosis is a rare complication of poorly controlled diabetes mellitus, of which pathomechanism is still not fully understood. In this report we discussed the clinical features, diagnostic processes and exam results of two elderly patients who admitted to our institution complaining of severe leg pain.
CASE
1. Case 1
A 90-year-old female was admitted to our hospital presenting with a 3-month history of bursting and burning left leg pain. She had been diagnosed with type II diabetes mellitus (DM) and DM-related polyneuropathy 2 years ago. Her glucose levels were well controlled (admission HbA1c level, 6.7%). She also had a history of ischemic stroke, atrial fibrillation, and coronary artery disease, for which she was taking anticoagulants. She had no history of leg trauma.
Her leg pain was accompanied by edema and tenderness around the knee and ankle, and her C-reactive protein (CRP) level was elevated (6.7 mg/dL). Creatine kinase and myoglobin levels were normal. Due to the clinical symptoms implying an inflammatory component, septic arthritis, gouty arthritis, and cellulitis were first considered differential diagnoses, rather than aggravation of DM-related polyneuropathy. Empirical ceftriaxone, non-steroidal anti-inflammatory drugs (NSAIDs), and colchicine were started concomitantly; however, they did not ameliorate her leg pain. Aspiration was performed to extract a small amount of left knee joint fluid, revealing a low white blood cells (WBC) count, but insufficient to indicate sepsis, and no crystalloids. She underwent a leg Doppler sonography to help determine the location and nature of her leg pain, and a deep vein thrombosis was ruled out.
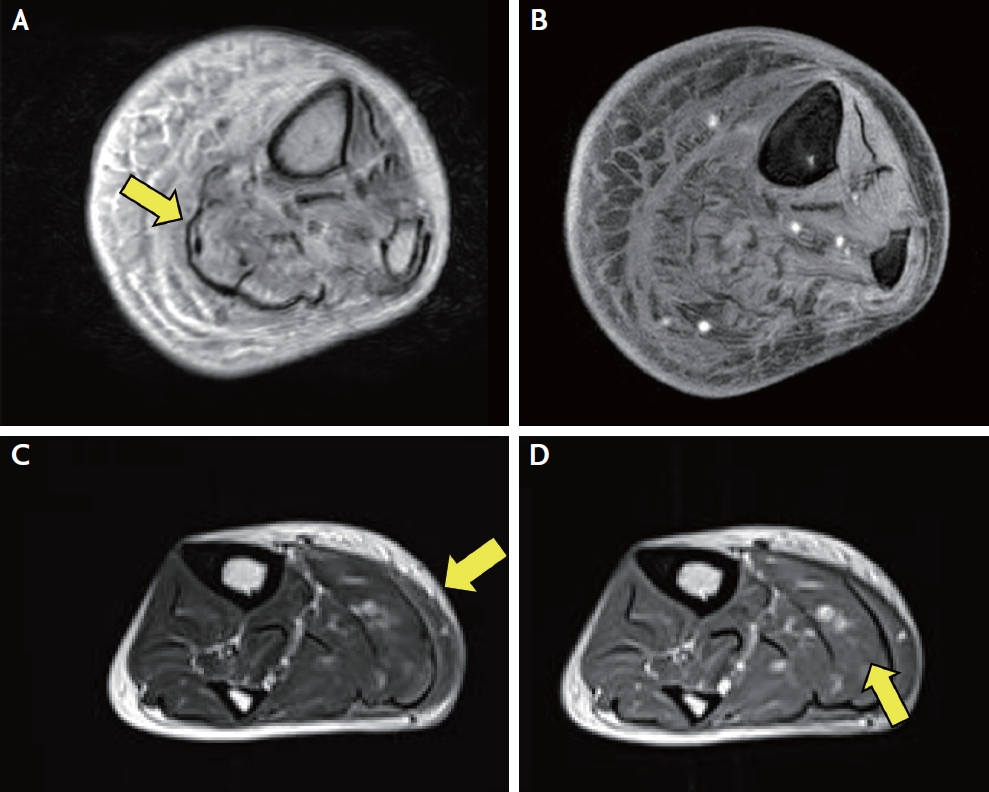
Leg magnetic resonance imaging (MRI) revealed diffuse edema and fatty degeneration of the calf muscles, particularly in the posterior muscle compartment. Diffuse thickening of the skin and subcutaneous fat at the medial aspect of the lower leg was observed, without significant enhancement (Fig. 1A, B). Pain was managed with acetaminophen and NSAIDS, after which she slowly improved and was discharged. At a 4-week follow-up, our patient was pain-free without muscle weakness sequelae.
2. Case 2
An 80-year-old female presented with bilateral intolerable calf pain. Owing to her severe pain, motor power was not fully examined; however, proximal muscle power in the lower extremities and sensory examinations were normal. She had a 20-year history of type II DM, which had been poorly controlled over the past year (HbA1c, reaching as high as 12.5%), requiring insulin. She had DM-related nephropathy and a 3-year history of arteriosclerosis obliterans in the right leg prior to presentation. Angioplasty and stent insertion were performed in the right superficial femoral artery. She had been taking dual antiplatelet agents, which were arbitrarily stopped until 6 months ago. We suspected peripheral artery disease recurrence; however, her dorsalis pedis pulses were palpable bilaterally. Furthermore, computed tomography angiography and vascular sonography showed no arterial or deep venous occlusion in the legs.
Blood test results indicated mildly elevated myoglobin (82 ng/mL) and creatinine kinase (CK, 80 U/L) levels. Her initial WBC count was slightly elevated (12.43 × 103/UL), whereas her CRP level was normal (0.16 mg/dL). The erythrocyte-to-sediment ratio was evaluated on hospital day 7 and it was normal (16 mm/hr). Leg MRI showed muscle and fascial edemas with enhancement predominantly in the posterior compartment (Fig. 1C, D). Her pain slowly subsided over a month with opioid patches and NSAID administration, and she was discharged. During several follow-up outpatient clinic visits, she had no further pain or sequelae.
DISCUSSION
Isolated leg pain in elderly patients with DM prompts consideration of differential diagnoses such as DM-related polyneuropathy, DM-related amyotrophy, atherosclerosis obliterans, and septic joints. DMI is a further differential diagnosis to consider. Detailed history taking, regarding the time course and nature of pain and accompanying neurological examination, facilitates narrowing down the differential diagnoses.
In the aforementioned cases, the only symptom was calf pain without definite neurological deficits. A continuous bursting sensation and pressuring pain, rather than electric shock-like pain, suggest nociceptive rather than neuropathic pain. More specifically, a mild elevation in CK and myoglobin levels combined with compartment syndrome-like pressuring pain is indicative of muscle injury and edema. Leg MRI was the most useful technique to localize and evaluate the severity and extent of the pain.
An acute onset of leg pain suggests vascular, infectious, or inflammatory causes rather than metabolic or genetic causes. Ancillary tests, such as arterial imaging or joint fluid aspiration, were performed, which enabled us to eliminate various suspected etiologies. After we had excluded all other possibilities from the differential diagnoses, we were able to make a diagnosis of diabetic muscle ischemia.
While the pathomechanisms of DMI remain unknown, ischemia-reperfusion injury, vasculopathic changes, and hypercoagulability have been previously proposed.1 DMI predominantly, but not exclusively, affects the posterior compartment of the lower extremity muscles. Laboratory examinations are usually nonspecific; CK levels are normal in 70% of patients, while inflammatory markers, such as the erythrocyte sedimentation rate and CRP levels, are elevated in 80% and 90% respectively.2
MRI is sensitive and specific for detecting DMI with high signal intensities in the T2 sequence and iso-to hypointensities in the T1 image.3 Moreover, necrosis or hemorrhage can be observed in the T1 sequence. Perimuscular and subcutaneous edemas are often accompanied with severe DMI. Additionally, diffuse heterogeneous enhancement may be observed on the post-gadolinium scan. Infectious myositis can also cause muscle edema with enhancement, but it is more localized and commonly presents as an abscess. In contrast, inflammatory myositis is usually symmetric and involves the proximal muscles.
In one systematic review, the most affected regions were the anterior thigh muscles, particularly the vastus medialis and lateralis, followed by the calf and back thigh muscles.2 In this case report, both patients showed posterior compartment predominant calf lesions on MRI. Some known myopathies that predominantly affect the posterior compartment include Miyoshi myopathy, desminopathy, vocal cord and pharyngeal myopathy.4 However, considering the age of the patients, this genetic etiology was unlikely.
Management of DMI remains supportive care, focusing mainly on bed rest, pain control, and rigorous glycemic control. Supportive care with bed rest and NSAIDs resulted in the shortest recovery time (28.5 days), whereas physical therapy prolonged symptom resolution to 76.5 days. Surgical intervention is generally not recommended as it increases the recovery time to 91 days.2
A typical clinical picture of DMI is that of an older adult patient with poorly controlled DM presenting with acute and severe thigh pain and nonspecific laboratory results. However, DMI should not be hastily excluded even when a patient presents with an atypical clinical picture, as shown in our two cases, with variations in the clinical presentation: the distribution of DMI (one vs. two legs), DM status (well-controlled recently diagnosed vs. poorly controlled long-standing DM), and laboratory results (elevated CRP but normal CK and myoglobin levels vs. normal CRP but elevated CK and myoglobin levels). In addition, patients are mostly older adults and experience severe pain, which make obtaining a detailed history challenging. Thus, it is important to perform examinations promptly to rule out a possible diagnosis of DMI and to perform leg MRI. As there is no specific treatment for DMI other than symptom control, patients can avoid undergoing unnecessary tests such as muscle biopsy or inappropriate management such as antibiotic treatment.